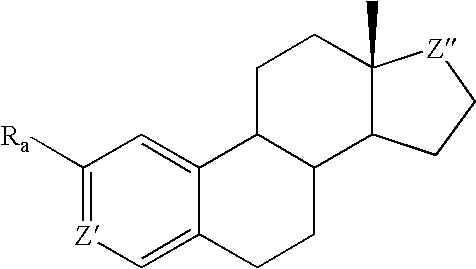
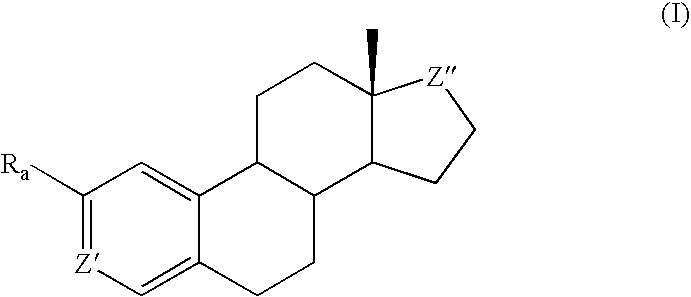
Methods of treating disease states using antiangiogenic agents
a technology of angiogenesis and disease state, applied in the field of angiogenesis treatment of disease state, can solve the problem that the estrogenic 2-hydroxyl derivative cannot be demethylated, and achieve the effect of improving absorption, transport, and reducing toxicity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
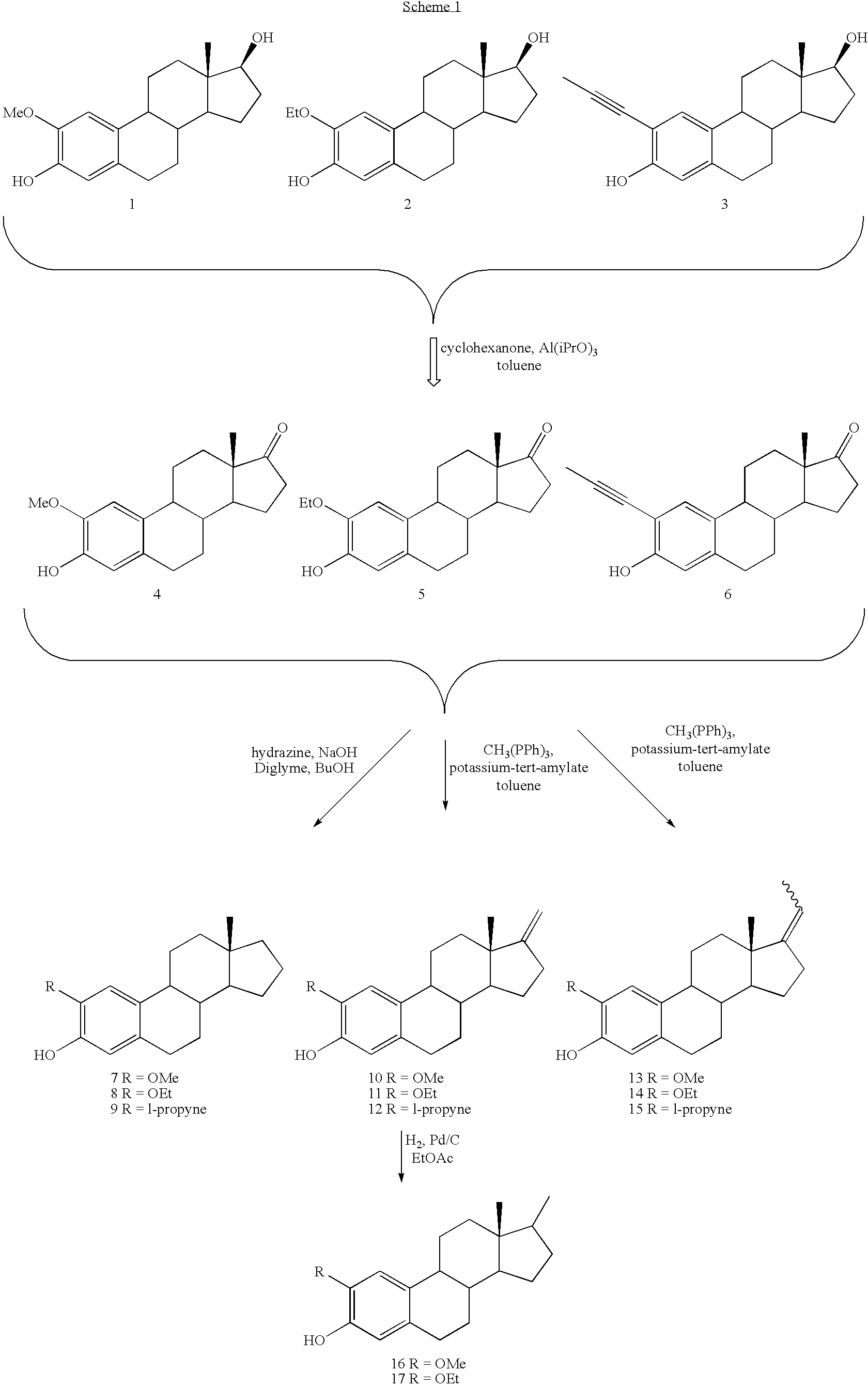
[0127] The procedures described below are for specific compounds. However, these reactions can be applied to all examples in this patent by one skilled in the art. References to compound #s below correspond to the numbers assigned to the compounds shown in the synthesis Schemes 1-7 above.
[0128] Representative oxidation of estradiol analogs to estrone analogs: 2-ethoxyestra-1,3,5(10)-trien-3-ol-17-one (Compound #5): 2-Ethoxyestra-1,3,5(10)-trien-3,17-diol (#2, 1.88 g, 5.67 mmol) was placed in a 250 mL round bottom flask that was equipped with a 25 mT Dean-Stark trap and a reflux condenser. The entire apparatus had been flame dried under an argon atmosphere. Toluene (50 mL) was added to dissolve the starting material. Aluminum isopropoxide (5.7 g, 28.4 mmol) and cyclohexanone (23.5 mL, 2.26.8 mmol) were added and the entire reaction mixture was heated at reflux (145-150° C.) for 20 h. Saturated aqueous sodium bicarbonate solution (100 mL) was added after the reaction mixture was allo...
example 2
[0167] Determination of in vitro anti-proliferative activity of substituted estradiol analogs: In vitro anti-proliferative or anti-mitogenic activity was determined using a commercially available cell-based assay in 96-well tissue culture plates with assessment of proliferation by evaluating DNA synthesis through incorporation into DNA of immuno-reactive (BrdU) nucleotides. The cell types used are commercially available (MDA-MB-231: breast cancer; U87-MG: glioblastoma; PC3: prostate cancer; HUVEC: non-transformed early passage human umbilical vein endothelial cells). These assays, and the many assay variations possible to determine in vitro anti-proliferative or anti-mitogenic activity, are well known to those skilled in the art. The concentration which causes 50% inhibition of proliferation (IC50) was estimated from a does-response curve generally carried out with a range of concentrations from ≧100 microg / mL to ≦0.01 microg / mL. The results of the tests are shown below in Table IV....
PUM
| Property | Measurement | Unit |
|---|---|---|
| volume | aaaaa | aaaaa |
| diameter | aaaaa | aaaaa |
| size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com