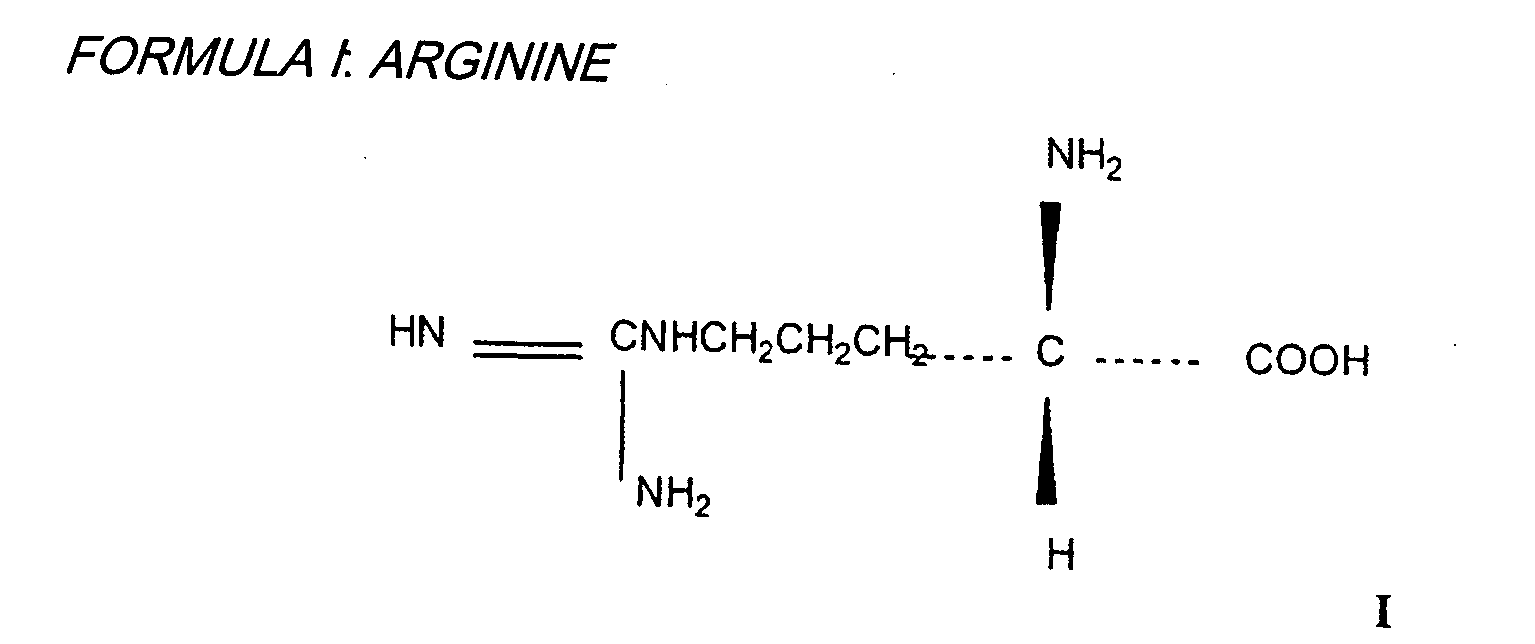
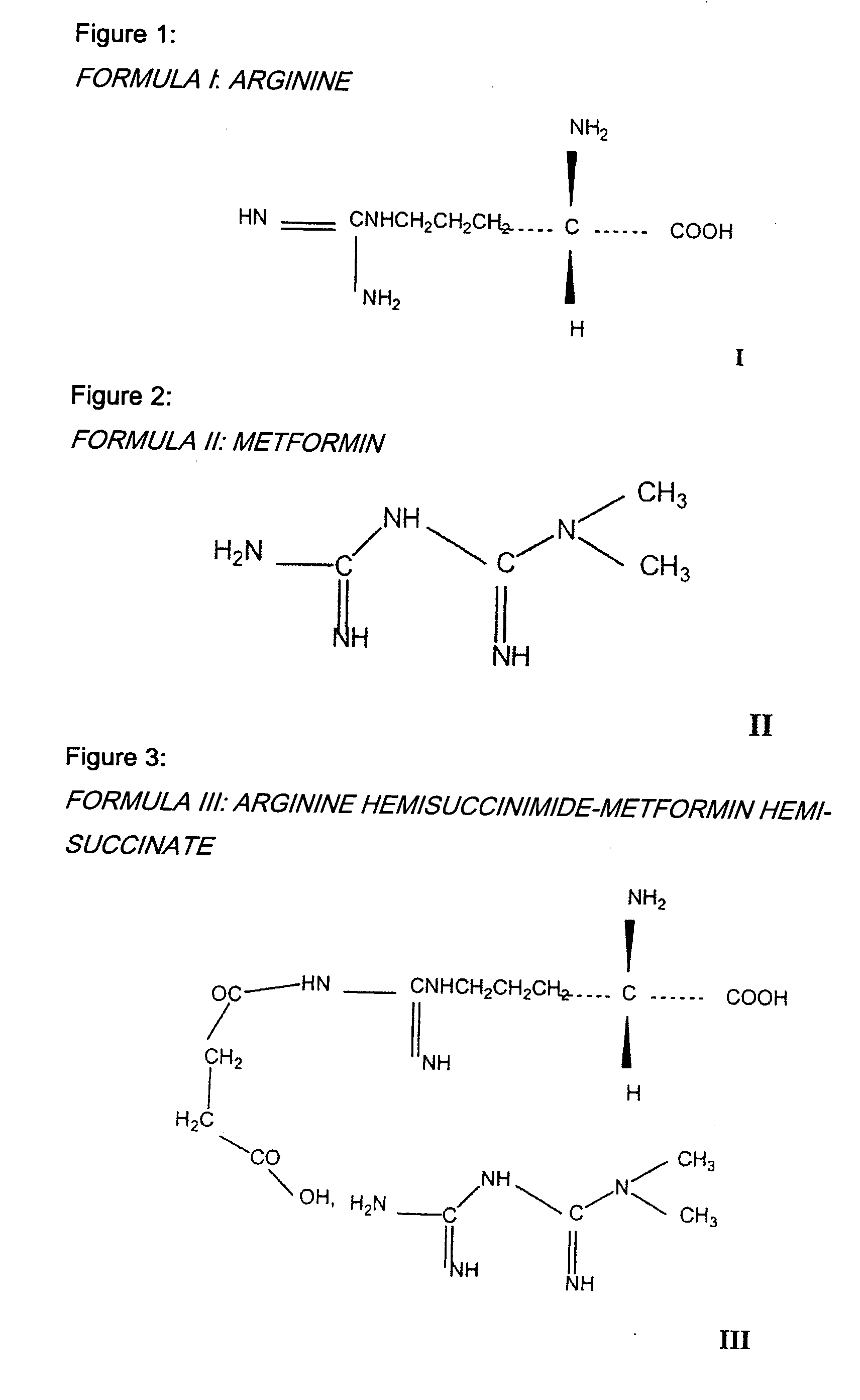
Medicinal association of a biguanine and a carrier, for example metformin and arginine
a biguanine and carrier technology, applied in the field of medicine combination, can solve the problem of low bioavailability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
[0044] The expression “biguanide” is understood to mean in particular N-dimethylbiguanides, substituted or otherwise, and for example metformin, but also other pharmaceutical compounds, for example buformin or fenformin.
[0045] Preferably, the biguanide is metformin.
[0046] The expression “simultaneous administration” is intended to mean the administration, in a single dose, of the two active principles, it being understood that the simultaneous administration allows the release, in the organism, of the two active principles simultaneously or in sequence.
[0047] The term “biogenic” is intended to mean a chemical compound which is of natural or unnatural origin and / or is metabolizable and / or is biodegradable and / or is atoxic with respect to the human or the animal, at a physiological dose.
[0048] The term “transporter” is intended to mean a molecule or substance which allows the transfer of another molecule across a barrier, either by forming an attachment, or without forming an atta...
PUM
| Property | Measurement | Unit |
|---|---|---|
| melting point | aaaaa | aaaaa |
| concentration | aaaaa | aaaaa |
| concentration | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com