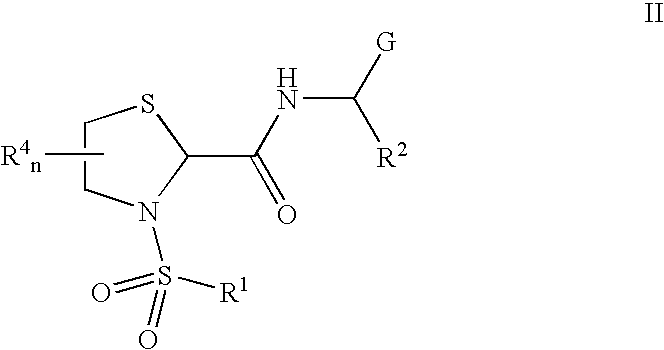
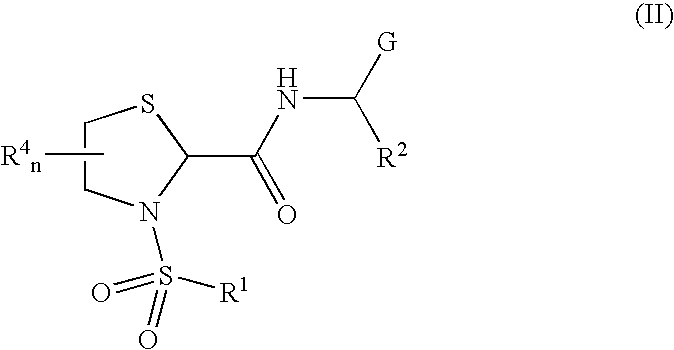
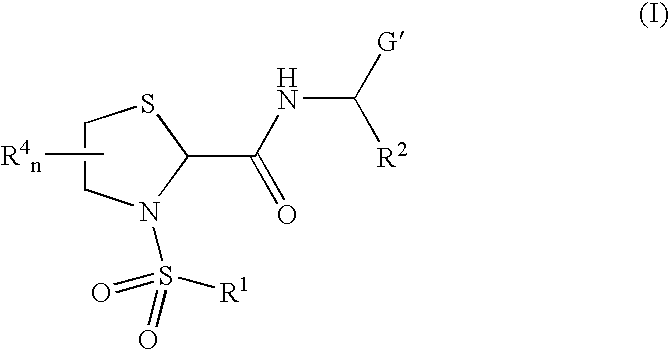
Thiazolidine carboxamide derivatives as modulators of the prostaglandin f receptor
a technology of prostaglandin f and derivatives, which is applied in the direction of heterocyclic compound active ingredients, biocide, drug compositions, etc., can solve the problems of preterm labor, unsuitable agents, and produce a corresponding reduction in the incidence of fetal respiratory distress
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
General protocols for the solution-phase synthesis of 1,3-thiazolidine-2-carboxamide derivatives of general formula (1); e.g., 3-([1,1′-biphenyl]-4-ylsulfonyl)-N—[(1S)-3-hydroxy-1-phenylpropyl]-1,3-thiazolidine-2-carboxamide; (2S)-3-([1′-biphenyl]-4-ylsulfonyl)-N-[(1S)-3-hydroxy-1-phenylpropyl]-1,3-thiazolidine-2-carboxamide; (2R)-3-([1,1′-biphenyl]-4-ylsulfonyl)-N-[1S)-3-hydroxy-1-phenylpropyl]-1,3-thiazolidine-2-carboxamide, (2S)-3-(1,1′-biphenyl-4-ylsulfonyl)-N—[(R)-phenyl(pyridin-2-yl)methyl]-1,3-thiazolidine-2-carboxamide
Strategy 1:
[0481]N-methyl morpholine (NMM) (3.24 g, 2.5 eq, 32.15 mmol) was added to a solution of a compound of general formula (VIII) (Intermediate 8, 4.50 g, 1 eq, 12.86 mmol), e.g., 3-([1,1′-biphenyl]-4-ylsulfonyl)-1,3-thiazolidine-2-carboxylic acid, in dry THF (100 ml) and the reaction mixture was cooled down to −25° C. To the reaction mixture was then added drop wise, over a period of 5 minutes, isobutyl chloroformate (1.84 g, 1.05 eq, 13.50 mmol) in solu...
example 2
3-([1,1′-biphenyl]-4-ylsulfonyl)-N-[(1R)-2-hydroxy-1-phenylethyl]-1,3-thiazolidine-2-carboxamide
[0490]Following the general strategies and protocols outlined in Example 1, starting from 3-([1,1′-biphenyl]-4-ylsulfonyl)-1,3-thiazolidine-2-carboxylic acid (Intermediate 8) and commercial (2R)-2-amino-2-phenylethanol, the title compound was obtained in 98% purity by HPLC.
[0491]1H NMR (300 MHz, CDCl3); 2.4-2.9 (m, CH2S, 2H), 3.5-3.7 (m, CH2N, 2H), 3.7-3.9 (m, CH2O, 2H), 4.9 (m, CH, 1H), 5.2 (s, CH, 1H), 7.1-7.9 (m, CH(Ar), 14H); M+(ESI+): 469.2; M−(ESI−) 467.1.
example 3
3-([1,1′-biphenyl]-4-ylsulfonyl)-N—[(R-phenyl(2-pyridinyl)methyl]-1,3-thiazolidine-2-carboxamide
[0492]Following the general strategies and protocols outlined in Example 1, starting from 3-([1,1′-biphenyl]-4-ylsulfonyl)-1,3-thiazolidine-2-carboxylic acid (Intermediate 8) and (R)-phenyl(2-pyridinyl)methanamine (Intermediate 1), the title compound was obtained in 96% purity by HPLC.
[0493]1H NMR (300 MHz, CDCl3); 2.5-3.0 (m, CH2S, 2H), 3.6-4.0 (m, CH2N, 2H), 5.41 (s, CH, 0.5H), 5.42 (s, CH, 0.5H), 6.07 (m, CH, 1H), 5.2 (s, CH, 1H), 7.1-7.8 (m, CH(Ar), 16H), 7.8-7.9 (m, CH, 1H), 8.5-8.6 (m, CH, 1H); M+(ESI+): 516.3; M−(ESI−) 514.1.
PUM
| Property | Measurement | Unit |
|---|---|---|
| concentrations | aaaaa | aaaaa |
| temperature | aaaaa | aaaaa |
| temperature | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com