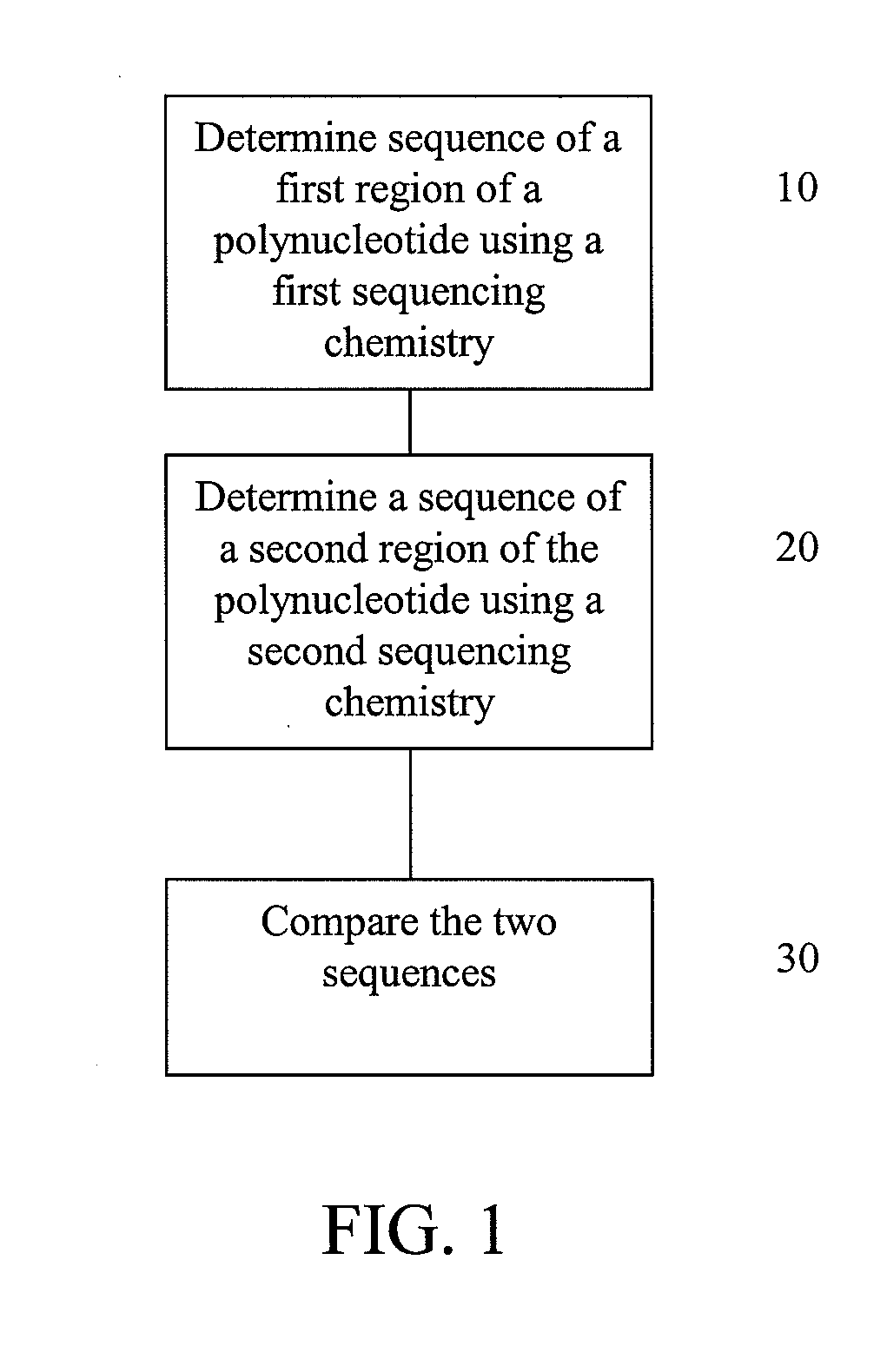
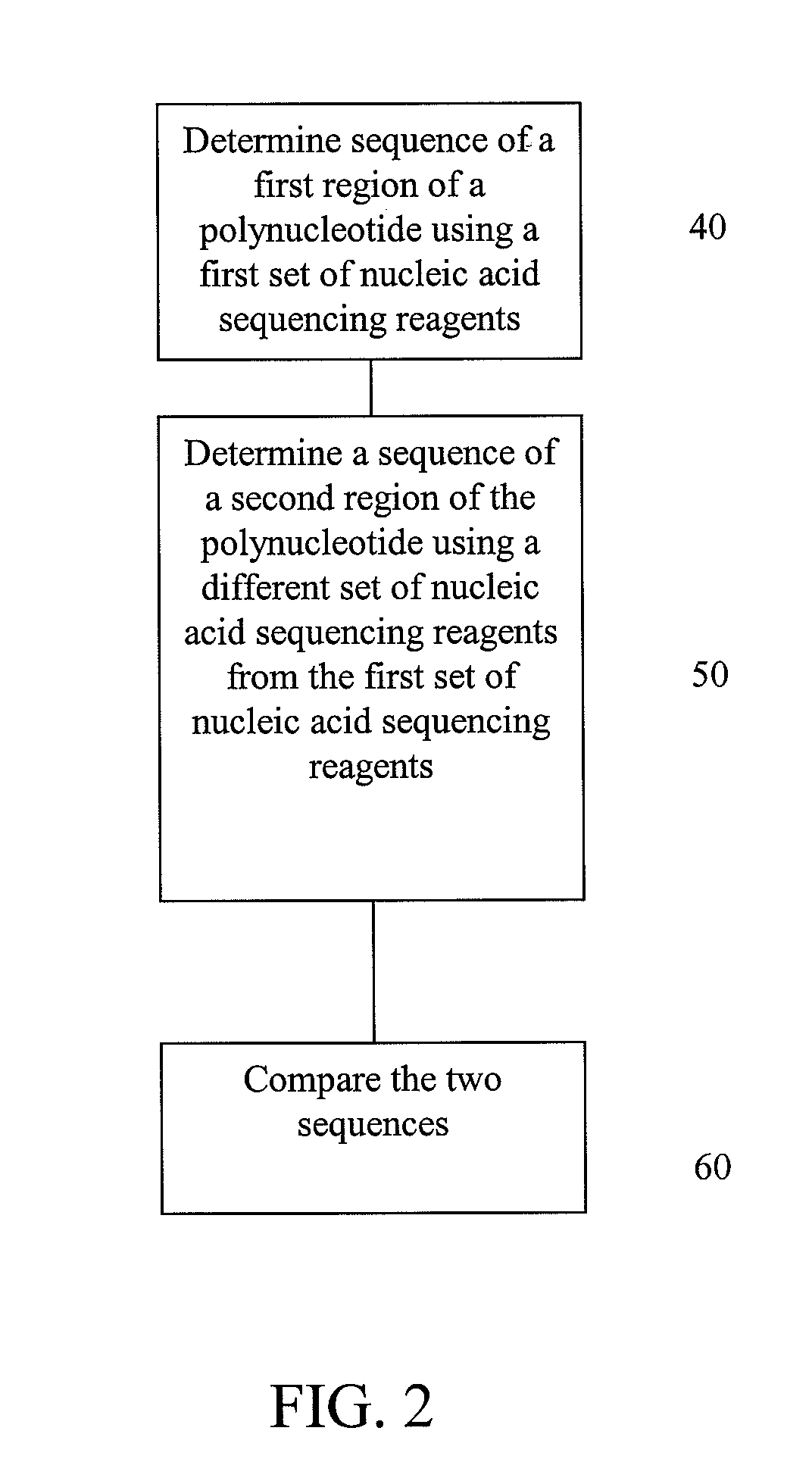
Alternative nucleic acid sequencing methods
a technology of nucleic acid and composition, applied in the field of methods and compositions for nucleic acid sequencing, can solve problems such as uncertainty in the obtained sequen
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
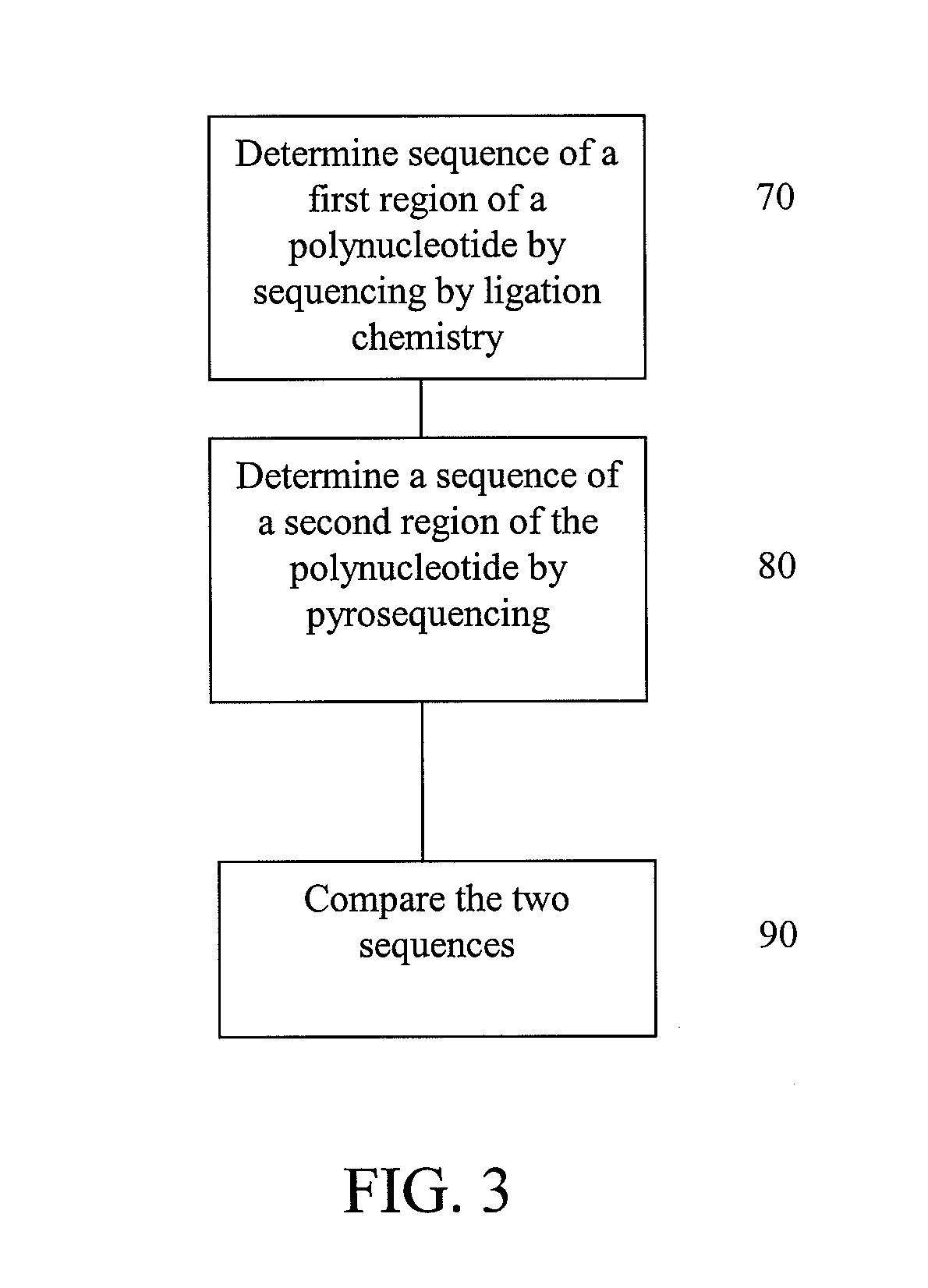
[0093]This example illustrates possible methods for analysis of a polynucleotide sequence by sequencing two regions of the polynucleotide using sequencing by ligation and pyrosequencing.
[0094]The polynucleotide for sequencing is prepared by fragmenting the polynucleotide and clonally amplifying the fragments through emulsion PCR as described below. After amplification, a first region of the polynucleotide is subjected to sequencing using sequencing by ligation, as described below, to determine a first sequence (FIG. 3 at 70). Next, a second region of the polynucleotide is subjected to pyrosequencing, as described below, using to determine a second sequence (FIG. 3 at 80). The first sequence is compared to the second sequence (FIG. 3 at 90). Concordance or discordance between the data from the two different chemistries can be taken into account when making a final base call for a given position. Variations in the base and sequence biases of the different chemistries may be also be ta...
example 2
[0095]This example illustrates possible methods for analysis of a polynucleotide sequence by sequencing two regions of the polynucleotide using sequencing by ligation and pyrosequencing.
[0096]The polynucleotide for sequencing is prepared by fragmenting the polynucleotide and clonally amplifying the fragments through emulsion PCR as described below. After amplification, a first region of the polynucleotide is subjected to sequencing using sequencing by ligation, as described below, to determine a first sequence. Next, a second region of the polynucleotide is subjected to sequencing using reversible terminators to determine a second sequence. The first sequence is compared to the second sequence. Concordance or discordance between the data from the two different chemistries can be taken into account when making a final base call for a given position. Variations in the base and sequence biases of the different chemistries may be also be taken into account when making a final sequence d...
example 3
[0097]This example illustrates possible methods for analysis of a polynucleotide sequence by sequencing two regions of the polynucleotide using sequencing by ligation and pyrosequencing.
[0098]The polynucleotide for sequencing is prepared by fragmenting the polynucleotide and clonally amplifying the fragments through emulsion PCR as described below. After amplification, a first region of the polynucleotide is subjected to sequencing using reversible terminators to determine a first sequence. Next, a second region of the polynucleotide is subjected to pyrosequencing, as described below, using to determine a second sequence. The first sequence is compared to the second sequence. Concordance or discordance between the data from the two different chemistries can be taken into account when making a final base call for a given position. Variations in the base and sequence biases of the different chemistries may be also be taken into account when making a final sequence determination.
PUM
| Property | Measurement | Unit |
|---|---|---|
| Fluorescence | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com