Method for manufacture of escitalopram
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
experiment 1
[0046
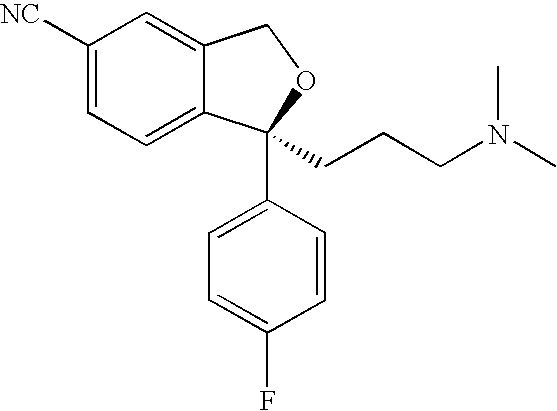
[0047](+)—O,O′-Di-p-toluoyl-(S,S)-tartaric acid (0.39 eq) was dissolved in 1-propanol (3.44 V). The mixture was heated up to ca. 40° C. and acetic acid (0.2 eq.) was added. This solution was transferred within one hour to a solution of 4-[4-(dimethylamino)-1-(4′-fluorophenyl)-1-hydroxybutyl]-3-(hydroxymethyl)-benzonitrile free base in 1-propanol (0.95 V) containing 0.1 V of toluene. The resolution mixture, containing now in total 4.4 V 1-propanol was seeded with seed crystals comprising S-4-[4-(dimethylamino)-1-(4′-fluorophenyl)-1-hydroxybutyl]-3-(hydroxymethyl)-benzonitrile and (+)—O,O′-di-p-toluoyl-(S,S)-tartaric acid and then stirred at 40° C. for 2 hours. The mixture was cooled to 20-25° C. within 2 hours. The product was filtered and washed twice with 1-propanol. The enantiomeric purity was typically in the range from about 91% to about 98% S.
[0048]The product was re-slurried in 1-propanol (2.5 V) at around 50° C. for 2 hours. The mixture was cooled to 20-25° C. The produc...
experiment 2
[0050
[0051](+)—O,O′-Di-p-toluoyl-(S,S)-tartaric acid (0.4 eq) was dissolved in 1-propanol (3.5 V). The mixture was heated up to ca. 40° C., acetic acid (0.2 eq.) was added and then the solution is transferred to a solution of 4-[4-(dimethylamino)-1-(4′-fluorophenyl)-1-hydroxybutyl]-3-(hydroxymethyl)-benzonitrile free base in 1-propanol containing 0.1 V toluene. The resolution mixture, containing now in total 4.5 V 1-propanol was seeded with seed crystals comprising S-4-[4-(dimethylamino)-1-(4′-fluorophenyl)-1-hydroxybutyl]-3-(hydroxymethyl)-benzonitrile and (+)—O,O′-di-p-toluoyl-(S,S)-tartaric acid and then stirred at 40° C. for two hours. The mixture was cooled to 20-25° C. in two hours. The product was filtered (filter reactor) and washed with 1-propanol.
[0052]The enantiomeric purity was typically around 97% S or higher.
[0053]An exemplary batch gave molar yield: 33.8%, enantiomeric purity: 99.0% S.
experiment 3
[0054
[0055]The general procedure of Experiment 2 was applied, however 0.5 eq of (+)—O,O′-di-p-toluoyl-(S,S)-tartaric acid and 10V of 1-propanol were used. No toluene or acetic acid was present in the system.
[0056]An exemplary batch gave molar yield: 29.5%; enantiomeric purity: 99.2% S.
PUM
| Property | Measurement | Unit |
|---|---|---|
| Temperature | aaaaa | aaaaa |
| Temperature | aaaaa | aaaaa |
| Temperature | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com