Multidimensional mass spectrometry of serum and cellular lipids directly from biologic extracts
a serum and cellular lipid technology, applied in the field of multi-dimensional mass spectrometry of serum and cellular lipids directly from biologic extracts, can solve the problems of destroying or significantly reducing the quality of life, and the paucity of information on tg molecular species changes during pathophysiological alterations, etc., and achieve the effect of reducing the number of patients
Inactive Publication Date: 2009-05-28
GROSS RICHARD W +1
View PDF3 Cites 14 Cited by
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
This approach provides a rapid, reliable method for analyzing triglycerides, enabling detailed molecular species profiling and risk assessment for conditions like coronary artery disease, stroke, and obesity, and facilitating the identification of effective lipid-modulating drugs.
Problems solved by technology
Additionally, such afflictions destroy or significantly reduce the quality of life even if not immediately fatal.
Although some studies have measured total TG molecular species content in multiple different disease states, a paucity of information on TG molecular species changes during pathophysiological alterations is available.
Previous attempts at direct TG quantitation by positive-ion electrospray ionization mass spectrometry (ESI / MS) were undesirably confounded by the presence of overlapping peaks from choline glycerophospholipids requiring chromatographic separation of lipid extracts prior to ESI / MS analyses.
Moreover, isobaric molecular species present in all biological tissues prevent determination of individual molecular species of triglycerides from molecular weight determinations alone.
Method used
the structure of the environmentally friendly knitted fabric provided by the present invention; figure 2 Flow chart of the yarn wrapping machine for environmentally friendly knitted fabrics and storage devices; image 3 Is the parameter map of the yarn covering machine
View moreImage
Smart Image Click on the blue labels to locate them in the text.
Smart ImageViewing Examples
Examples
Experimental program
Comparison scheme
Effect test
examples
[0121]The present invention is more particularly described in the following Examples which are intended as illustrative only since numerous modifications and variations therein will be apparent to those skilled in the art. All weights and ratios used herein are on a weight basis unless otherwise specified.
Materials and Methods
the structure of the environmentally friendly knitted fabric provided by the present invention; figure 2 Flow chart of the yarn wrapping machine for environmentally friendly knitted fabrics and storage devices; image 3 Is the parameter map of the yarn covering machine
Login to View More PUM
| Property | Measurement | Unit |
|---|---|---|
| weight | aaaaa | aaaaa |
| internal average diameter | aaaaa | aaaaa |
| temperature | aaaaa | aaaaa |
Login to View More
Abstract
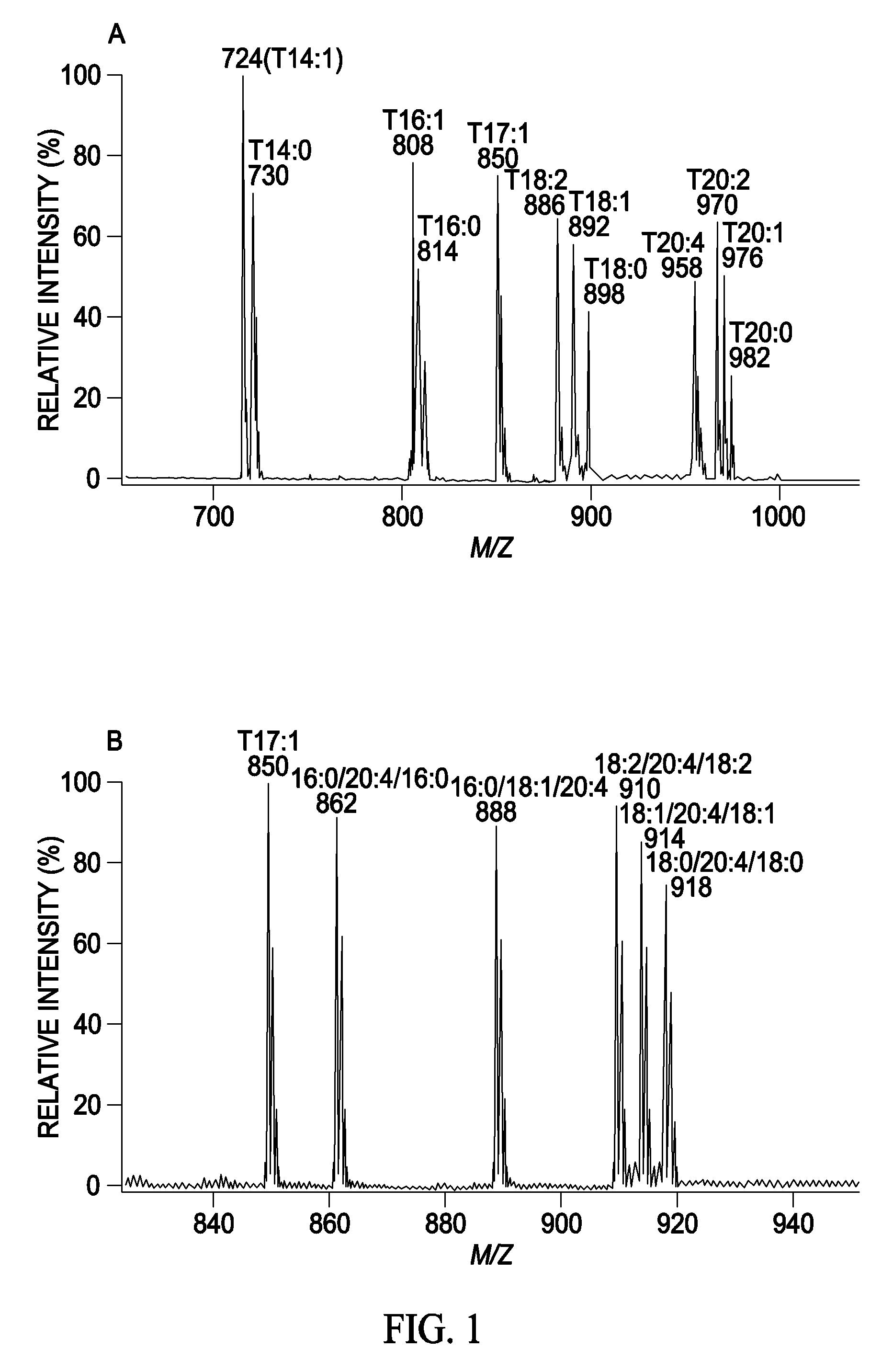
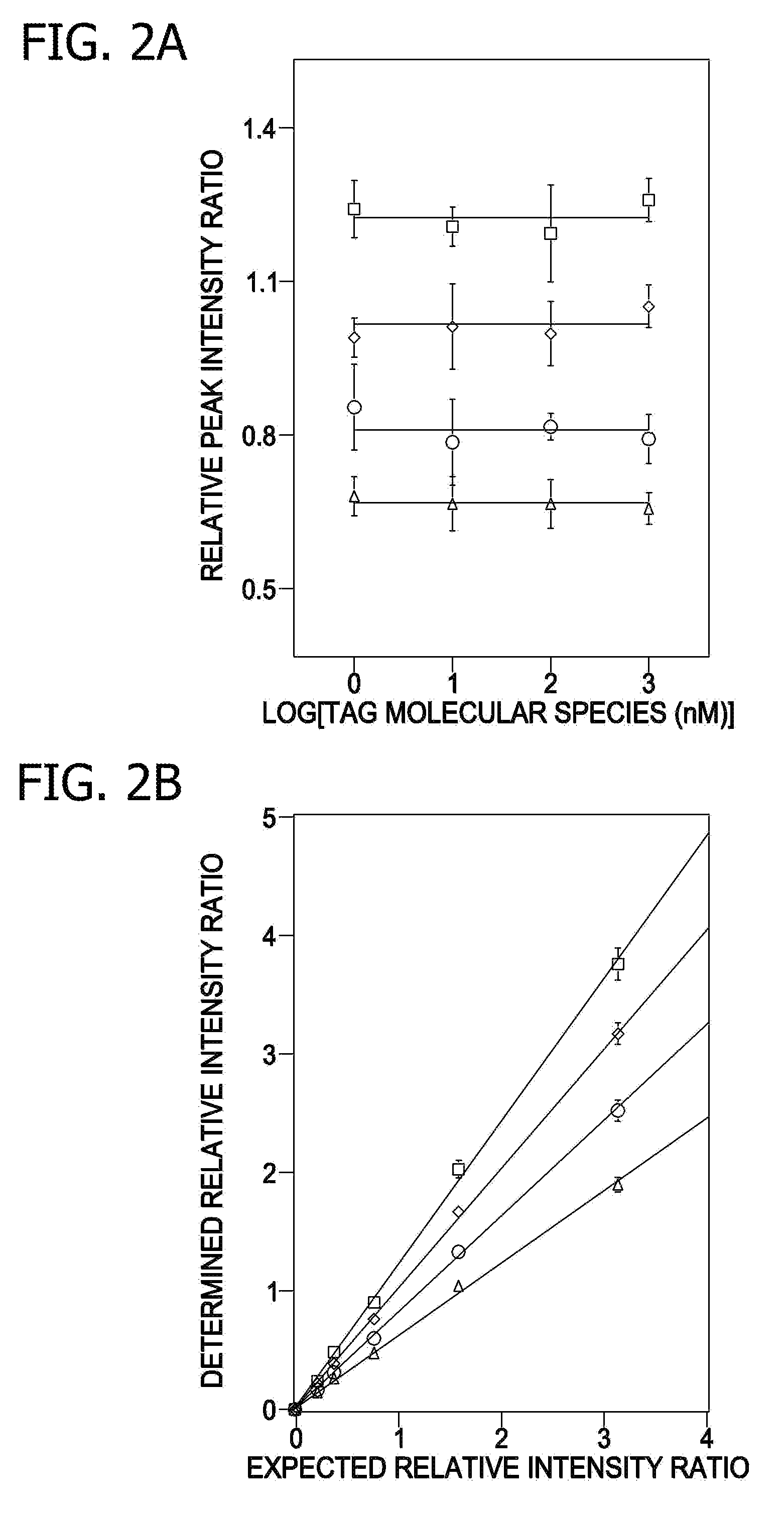
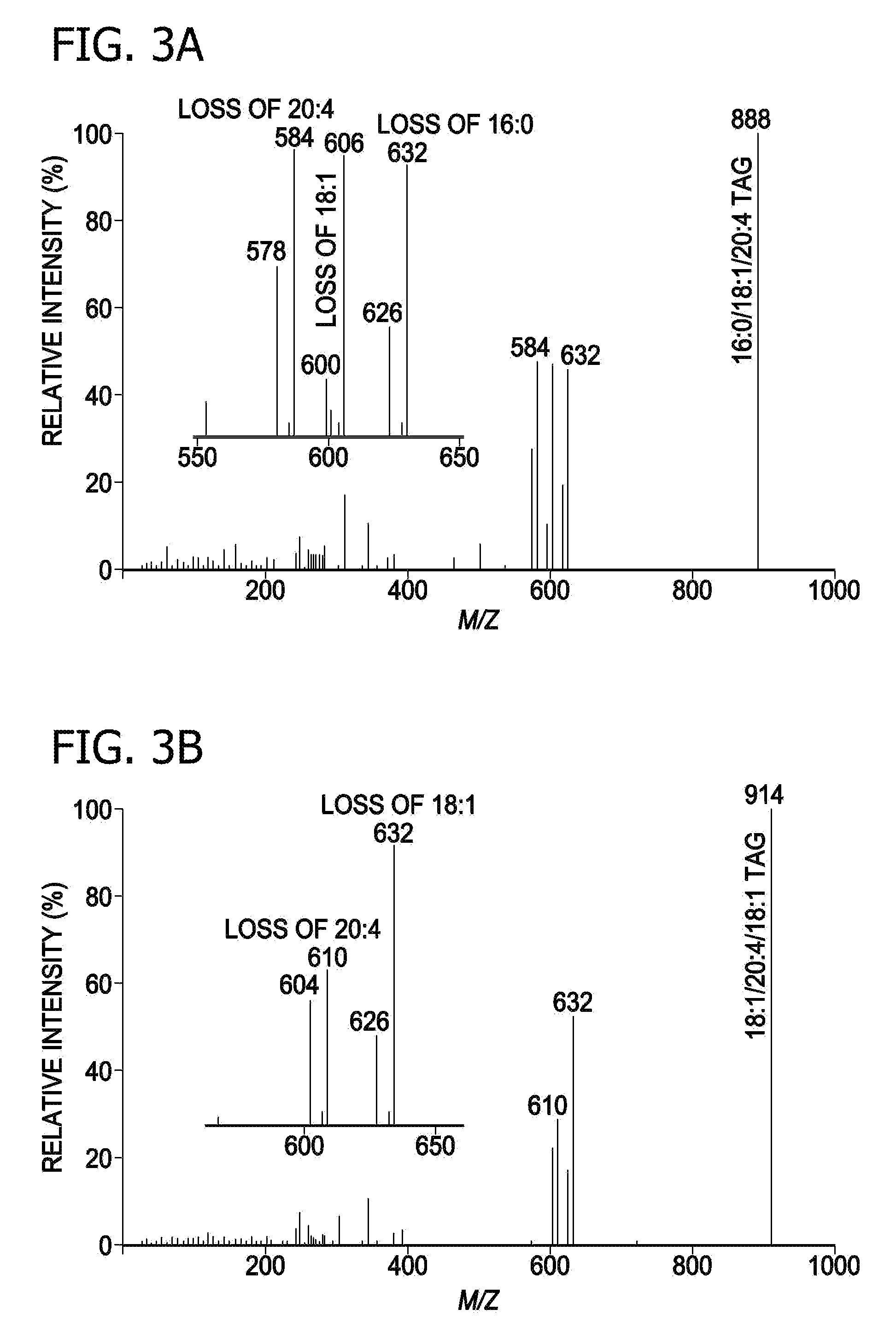
A method for determination of at least one of the lipid species in a biological sample comprising subjecting the sample to lipid extraction to obtain a lipid extract and subjecting the resulting lipid extract to multidimensional electrospray ionization mass spectrometry using either precursor ion or neutral loss scanning (or both) of all naturally occurring aliphatic chains, lipid fragments and precursor ions leading to observed fragments to generate a multidimensional matrix whose contour densities provides structural and quantitative information directly without chromatography. A method for determination of lipid content and / or lipid molecular species composition and quantity directly from lipid extracts of a biological sample comprising subjecting said lipid extract to electrospray ionization multidimensional mass spectrometry by comparisons to standards and algorithms described herein.
Description
CROSS REFERENCE TO RELATED APPLICATION[0001]This application is a divisional of U.S. nonprovisional patent application Ser. No. 10 / 797,616, filed Mar. 10, 2004, which is a continuation in part of pending U.S. nonprovisional patent application Ser. No. 10 / 606,601, filed Jun. 26, 2003, which has issued as U.S. Pat. No. 7,306,952 and which claims priority to U.S. provisional patent application 60 / 391,711 filed Jun. 26, 2002, and claims the benefit of U.S. provisional patent application 60 / 458,733 filed Mar. 28, 2003 all of which are incorporated by reference in their entirety.STATEMENT REGARDING FEDERALLY SPONSORED RESEARCH & DEVELOPMENT[0002]This work was supported by grants from NIH including grants W / H P01 HL57278 / JDFI 996003, R02HL41250, RO1 AA11094, P41-RR00954, P60-DK20579, and P30-DK56341. The government has certain rights in the invention.FIELD OF THE INVENTION[0003]This invention relates generally to a method of analysis for lipids including triglycerides and other mentioned c...
Claims
the structure of the environmentally friendly knitted fabric provided by the present invention; figure 2 Flow chart of the yarn wrapping machine for environmentally friendly knitted fabrics and storage devices; image 3 Is the parameter map of the yarn covering machine
Login to View More Application Information
Patent Timeline
 Login to View More
Login to View More Patent Type & Authority Applications(United States)
IPC IPC(8): B01D59/44G01N33/48G01N33/50G01N33/92G06F19/00
CPCG01N33/92G01N2800/044Y10T436/104165Y10S436/811Y10T436/24H01J49/00
Inventor GROSS, RICHARD W.HAN, XIANLIN
Owner GROSS RICHARD W
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com