B-cell reduction using cd37-specific and cd20-specific binding molecules
a b-cell and binding molecule technology, applied in the field of b-cell reduction, can solve the problems of insufficient b-cell activity, synergistic conjugations, inappropriate cell proliferation,
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
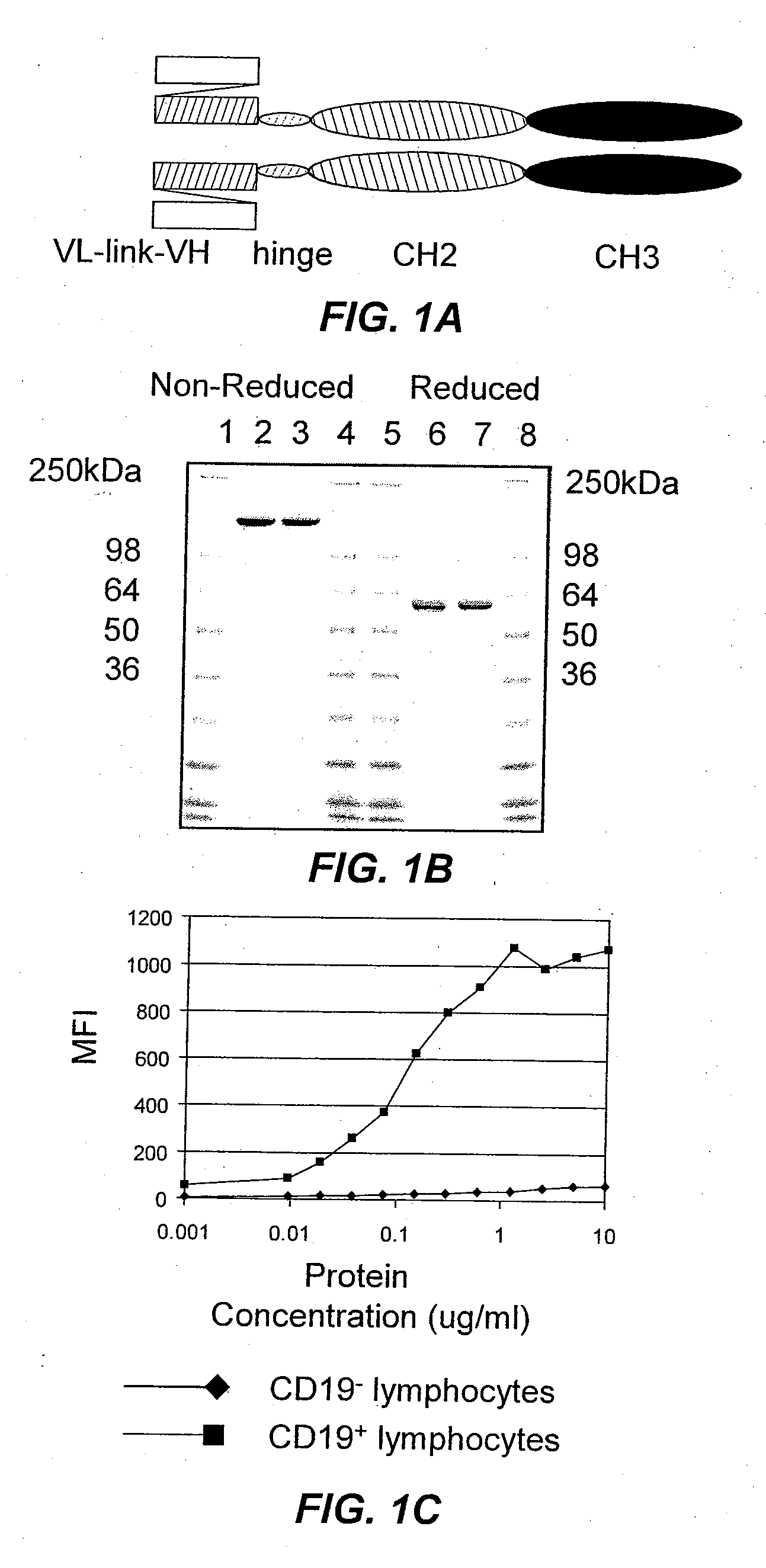
Production of a CD37-Specific Binding Molecule
[0174]CD37-specific SMIPs are described in co-owned U.S. application Ser. No. 10 / 627,556 and U.S. Patent Publication Nos. 2003 / 133939, 2003 / 0118592 and 2005 / 0136049. An exemplary SMIP, TRU-016, is produced as described below.
[0175]TRU-016 [G28-1 scFv VH11S(SSC-P)H WCH2 WCH3] is a recombinant single chain protein that binds to the CD37 antigen. The binding domain was based on the G28-1 antibody sequence previously disclosed in the patent publications listed in the preceding paragraph, which disclosure is incorporated herein by reference. The binding domain is connected to the effector domain, the CH2 and CH3 domains of human IgG1, through a modified hinge region. TRU-016 exists as a dimer in solution and the dimer has a theoretical molecular weight of approximately 106,000 daltons.
[0176]Total RNA from the G28-1 hybridoma was isolated using Trizol RNA (Gibco) reagent according to the manufacturer's instructions. cDNA was prepared using 5 μ...
example 2
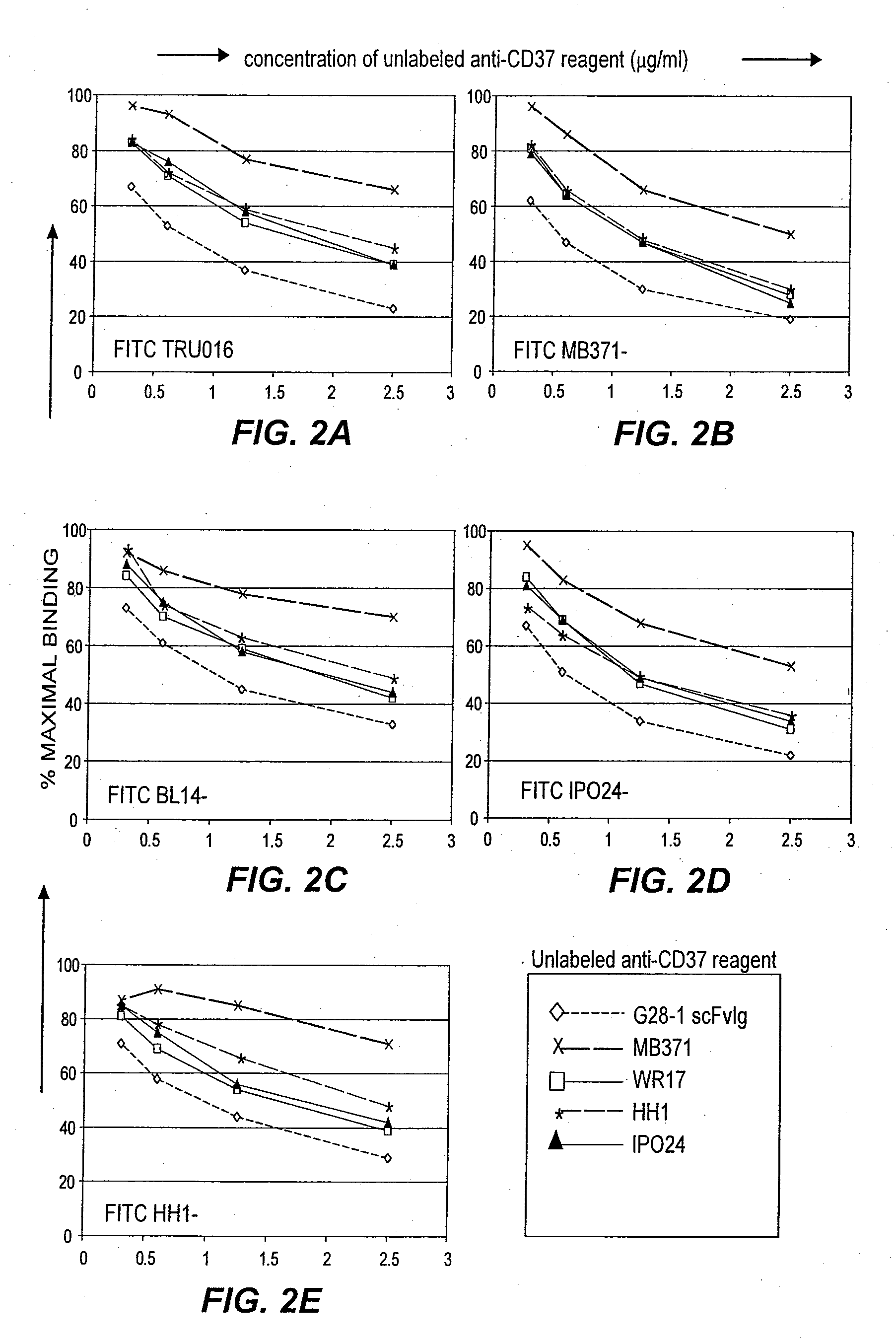
TRU-016 and Various CD37-Specific Antibodies Bind the Same or Overlapping Epitopes on CD37
[0186]Experiments were performed to identify the CD37 epitope bound by TRU-016 and other previously described CD37-specific antibodies.
[0187]Unconjugated MB371 (#555457) and FITC-conjugated MB371 (#555456) were obtained from BD Pharmingen (San Jose, Calif.), FITC-conjugated BL14 (#0457) from Immunotech / Beckman Coulter (Fullerton, Calif.), FITC-conjugated NMN46 (#RDI-CBL 136FT) and unconjugated NMN46 (#RDI-CBL 136) from RDI (Flanders, N.J.), FITC-conjugated IP024 (#186-040) and unconjugated IPO-24 (#186-020) from Ancell Corporation (Bayport, Minn.), FITC-conjugated HHI (#3081) and unconjugated HH1 (#3080) from DiaTec.Com (Oslo, Norway) and FITC-conjugated WR17 (YSRTMCA483F) and unconjugated WR17 (YSRTMCA483S) from Accurate Chemical & Scientific (Westbury, N.Y.). TRU-016 protein was produced as described in Example 1.
[0188]TRU-016 was conjugated to FITC at Trubion using a Molecular Probes Fluoror...
example 3
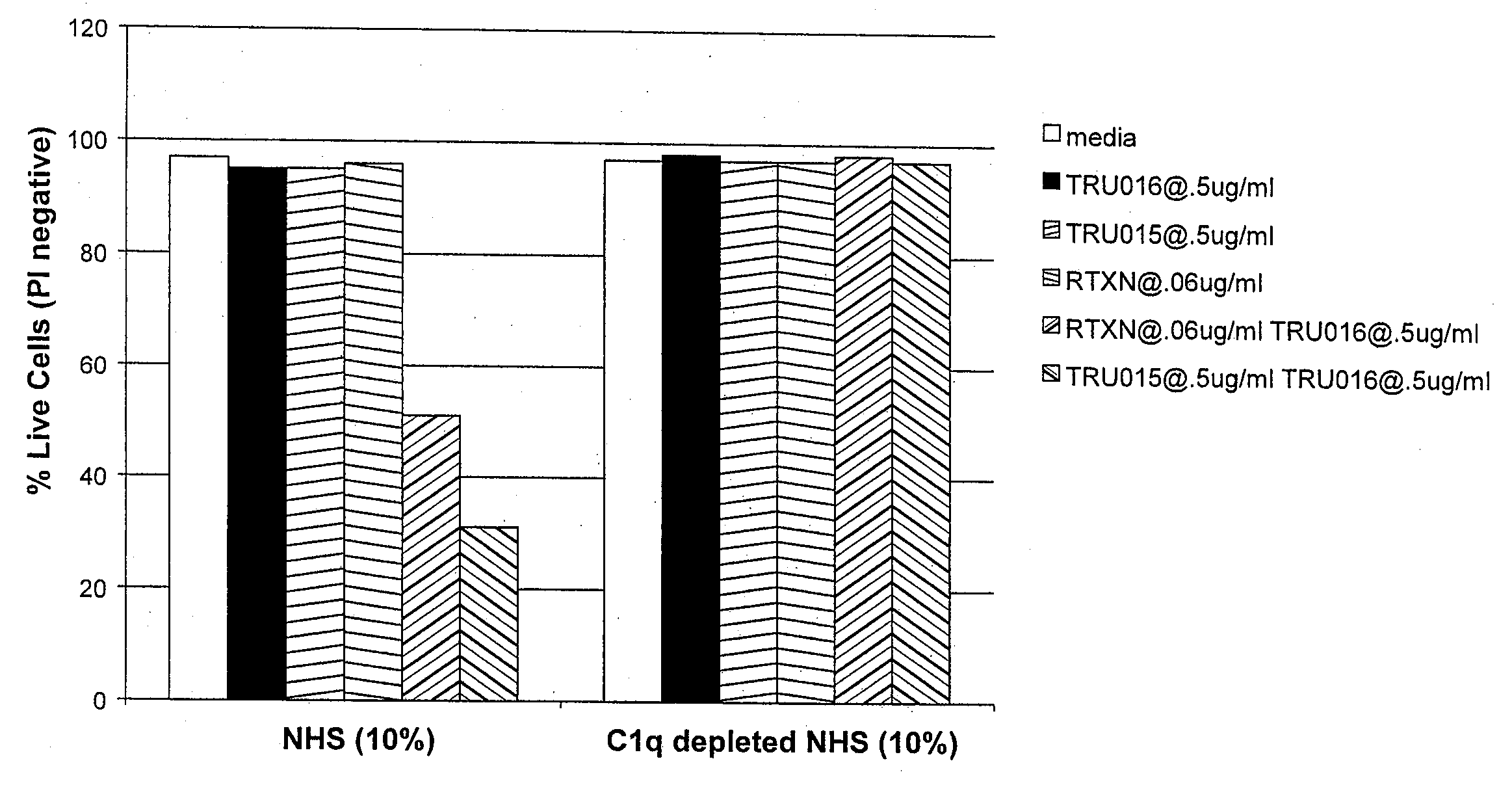
TRU-016 is Deficient in Binding C1q and Activating the Classical Complement Activation Pathway
[0198]Experiments were performed to explore why the TRU-016 dimer peak fails to mediate significant levels of complement dependent killing of B cell targets. One possibility was that TRU-016 dimer shows reduced binding to components of the complement cascade relative to normal human IgG1 antibody. Thus, experiments were performed to determine if TRU-016 activates the classical complement activation pathway by looking for TRU-016 binding to C1q. C1q, is a subunit of the C1 enzyme complex that activates the serum complement system, and is the recognition component of the classical complement activation pathway.
[0199]C1q binding studies were performed as previously described (Cragg et al., Blood 2004, 103:2738-2743). Briefly, Ramos B-cells in Iscoves media (#12440-053, Gibco / Invitrogen, Grand Island, N.Y.) with no serum were plated in 96-well V bottom plates at 5×105 / well in 100 μl. Cells were...
PUM
| Property | Measurement | Unit |
|---|---|---|
| half life | aaaaa | aaaaa |
| temperatures | aaaaa | aaaaa |
| molecular weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com