1,3-dipolar cycloaddition of azides to alkynes
a dipolar cycloaddition and azide technology, applied in the direction of adhesives, etc., can solve the problems of incomplete polymerization and reduced yield, and achieve the effects of reducing the dsc peak temperature, and increasing the loading
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
Curing Behavior of Azide and Alkyne Monomers in Bulk Phase without Catalysts
[0085]To get a better understanding of structure-cure temperature relationship, several structurally different alkynes were reacted in combination with dimer azide using DSC to react and cure the reactants. Tripropargylamine and nonadiyne were purchased from Aldrich; the other compounds were synthesized in-house. The results are reported in Table 1 and indicate that there is a strong dependence of cure temperature on the alkyne structure. All propargyl ethers, entries 1, 2, and 3, cured at 150° C. No significant effect of degree of branching of alkynes on cure temperature was observed (entries 1 and 2 compared to 3). In contrast to the propargyl ethers, the propargyl amines showed higher cure temperatures (entries 4 and 5). When the all-carbon alkyne, nonadiyne, was used, the cure temperature was the highest (entry 6).
[0086]The reactivity order of alkynes in the bulk phase uncatalyzed azide / alkyne chemistry ...
example 2
Catalytic Effect of Cu(I) Species in Bulk Phase Reactions
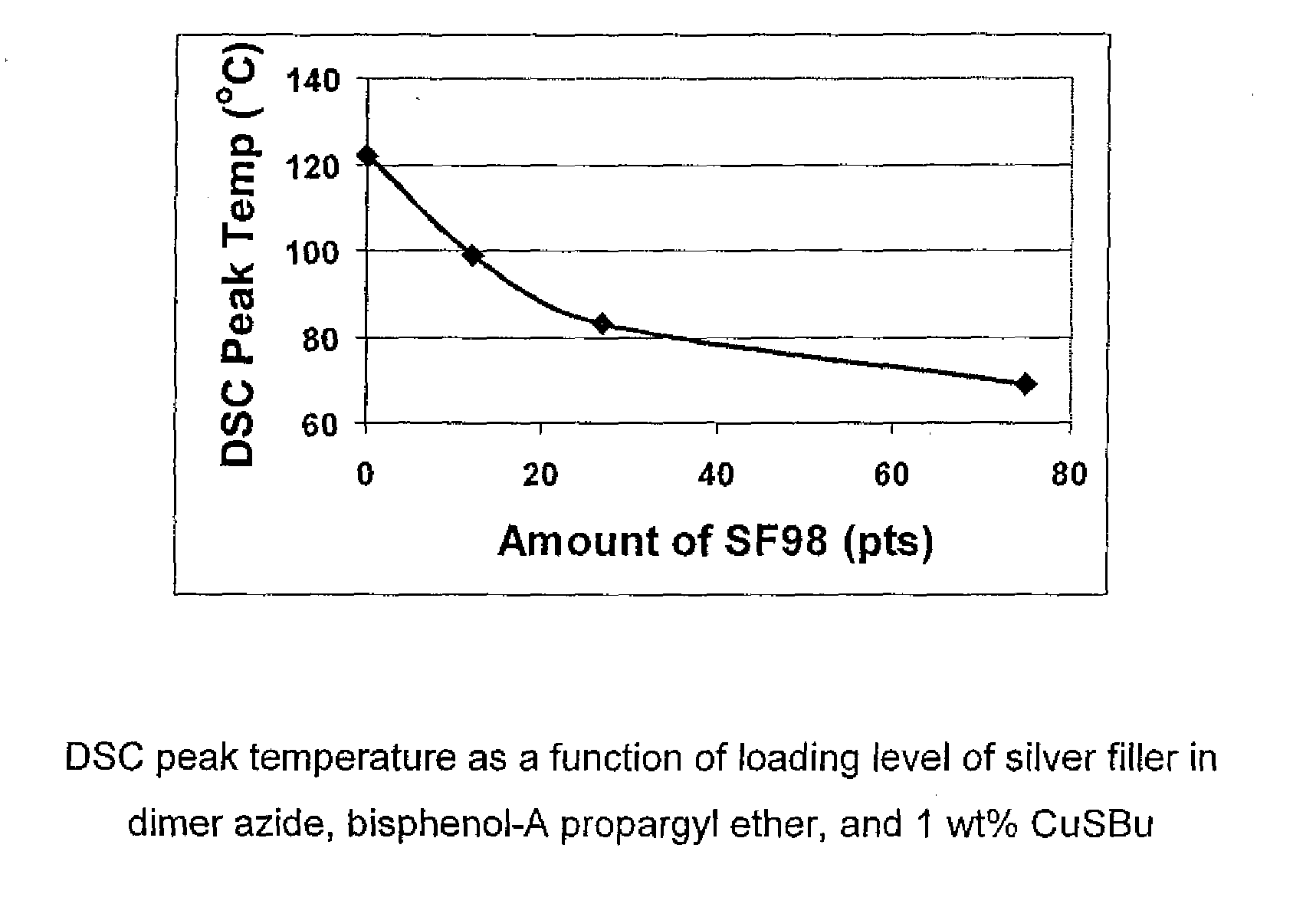
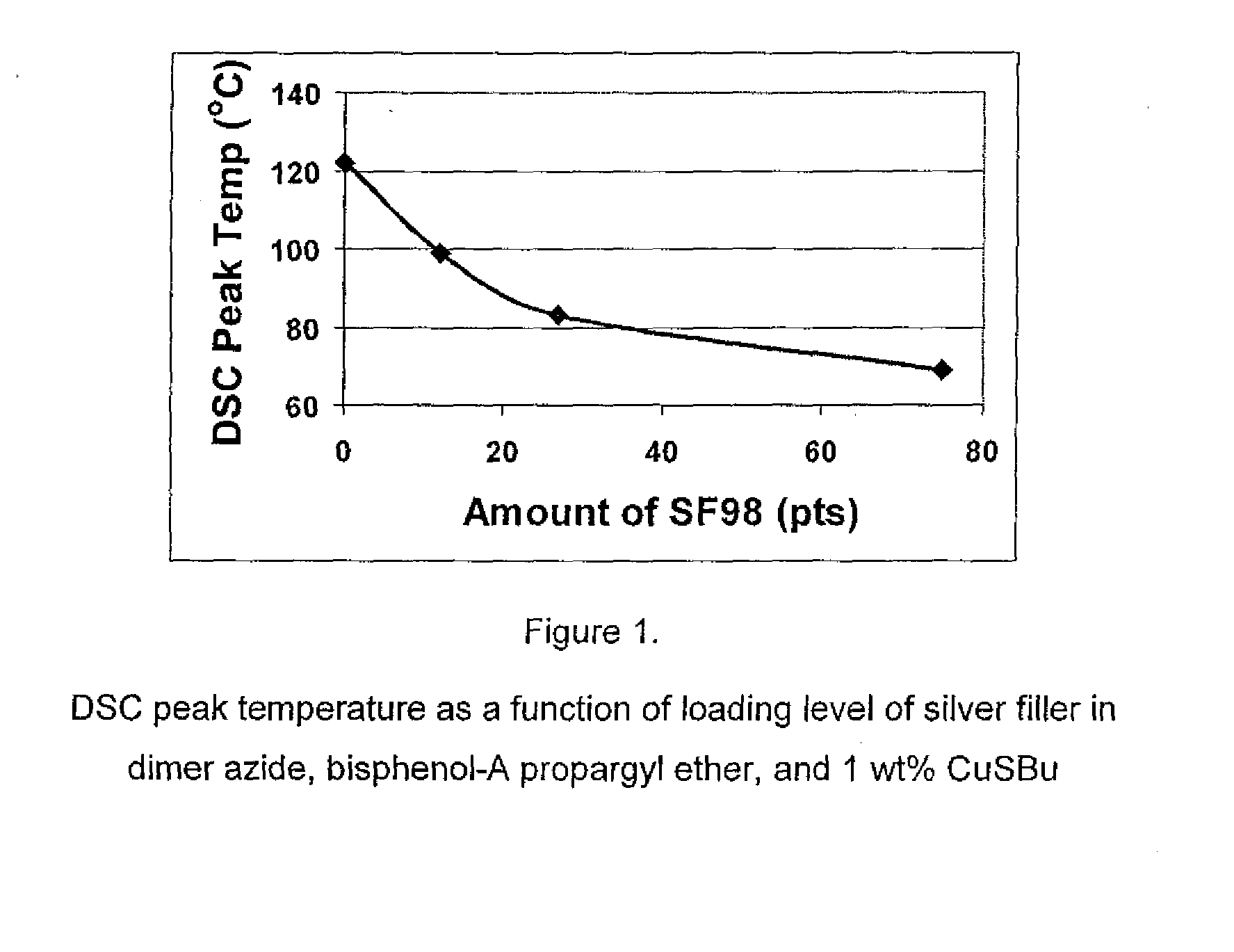
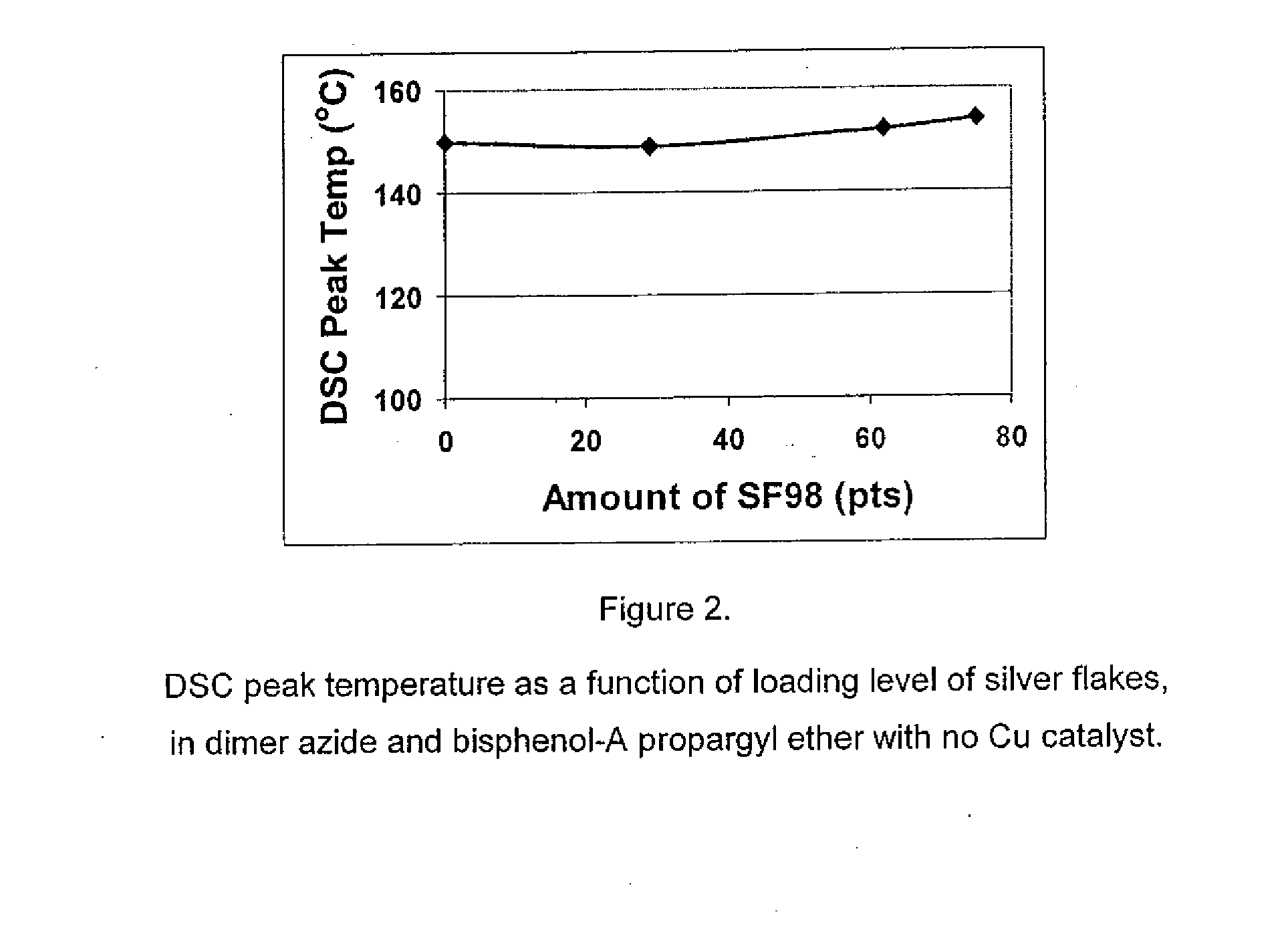
[0087]Three commercially available Cu(I) catalysts, CuI, CuSBu, and CuPF6(CH3CN)4, were screened to target a DSC peak temperature of approximately 100° C. compared to a control using no catalyst. The results are reported in Table 2. All of the catalysts used in the study decreased the DSC peak temperature of the formulations of entries 2, 3, 4, 10 compared to the control, entry 1; of the formulations of entries 6, 7 compared to the control, entry 5; the formulation of entry 9 compared to the control, entry 8. The magnitude of reduction in DSC peak temperature depended on the catalyst loading, entry 2 compared to entry 10, with higher loading giving the lowest peak temperature.
[0088]In addition to lowering the DSC peak temperatures, these Cu(I) catalysts also narrowed the cure profile considerably, making them more suitable for snap (fast) cure (see ΔT in entries 4, 6, 7, 9, compared with respective controls). With CuI and CuPF...
example 3
Effect of Cu(I) and Cu(II) Catalysts on Curing Temperature
[0089]Eight different Cu(I) catalysts and one Cu(II) catalyst without reducing agent were examined for their effect on the curing temperature of the azide / alkyne azide / alkyne chemistry using the same resin composition of dimer azide and bisphenol E propargyl ether in a 1:1 equivalent ratio with one weight % of the catalyst. Entry 1 is the control without catalyst, entries 2 to 9 are the Cu(I) catalysts, and entry 10 is the Cu(II) catalyst. Most Cu(I) catalysts significantly reduced the curing temperature (entries 2, 3, 4, 5, 6, 7), and some catalyzed the chemistry so dramatically that the resin composition gelled immediately after mixing at room temperature (entries 2 and 3, although no narrowing of DSC peaks were observed. The Cu(II) catalyst without a reducing agent unexpectedly also reduced the the curing temperature. The results are reported in TABLE 3.
TABLE 3EFFECT OF CU CATALYSTS ON CURING TEMPERATUREDSCTpeakTonsetΔTΔHE...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Temperature | aaaaa | aaaaa |
| Fraction | aaaaa | aaaaa |
| Temperature | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com