Forms of Prostate Specific Antigens and Methods for their Detection
a prostate specific and antigen technology, applied in the field of forms, can solve the problems of difficult development of ppsa-specific mabs, no suitable mabs,
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
Expression of pPSA in Mammalian Cells
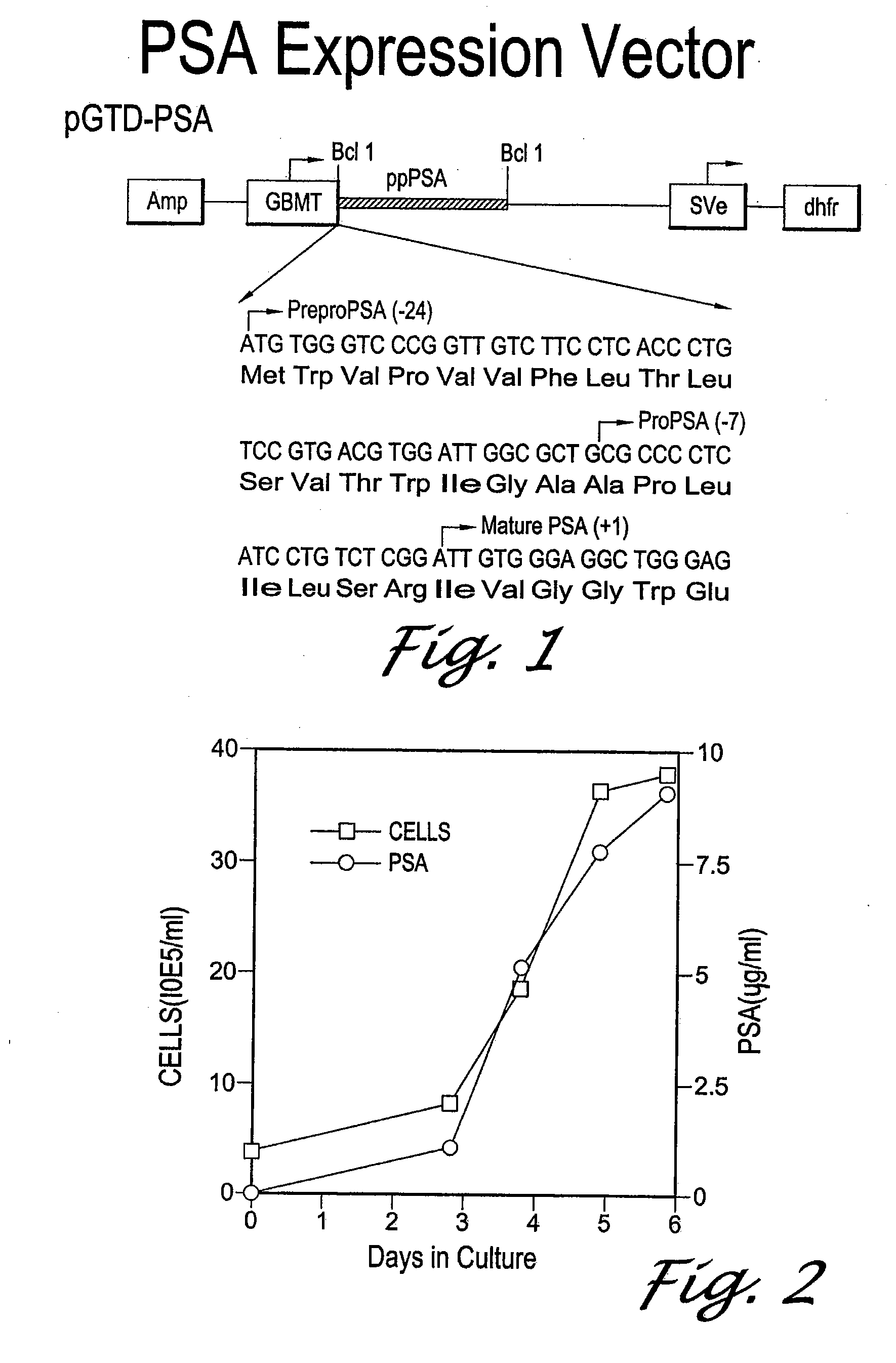
[0100]The cDNA for PSA was cloned into the pGT-d vector under the control of the GBMT promoter using an approach similar to the one described for hK2 by Kumar et al. To study the expression of PSA, AV12 cells were transfected with the pGTD-PSA expression vector. Cells were selected in 400 nM methotrexate for 2-3 weeks, and single cell clones were analyzed for PSA expression using TANDEM®-MP PSA assay and on Western blots using mAb PSM 773. Clone AV12-PSA#8 was selected based on its high expression levels of a PSA-immunoreactive band at ˜32 kDa.
[0101]To determine the PSA expression pattern in mammalian cells, samples of spent media from AV12-PSA#8 cells were collected for six consecutive days and analyzed using the TANDEM®-MP PSA assay. FIG. 2 shows that PSA was detected in spent media at day 1 and accumulated to >9 μg / ml by day 6. Expression of PSA was higher during the log phase of cell growth, indicating that a stable form of PSA is secreted by...
example 2
[0104]Detection of pPSA in Pooled Human Prostate Cancer Serum
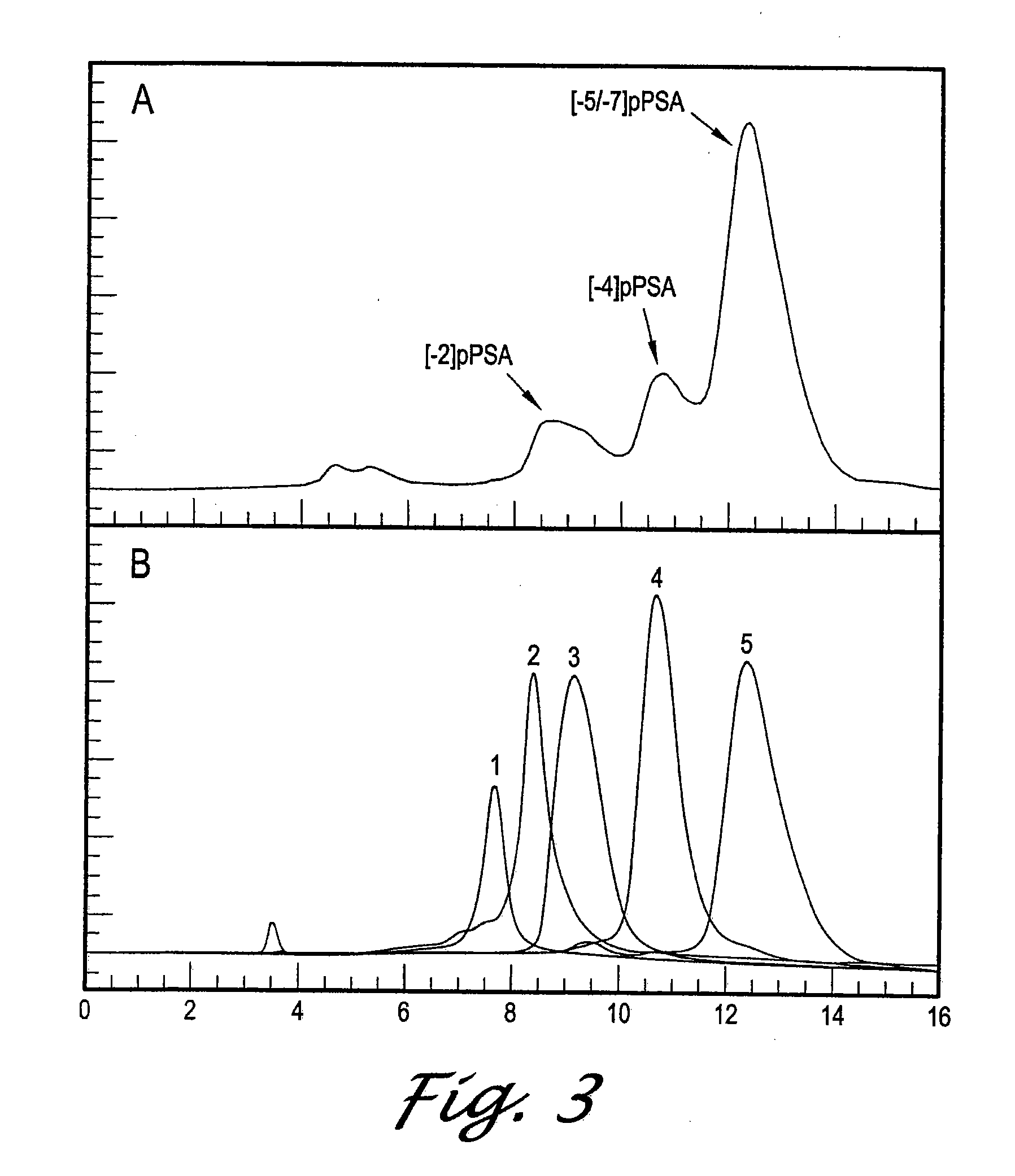
[0105]The presence of pPSA in human serum would indicate the following. First, that PSA is secreted as the pPSA form in human tissue and is converted to mature PSA extracellularly. Second, that pPSA is stable in human serum and thus may be a useful diagnostic marker for pCa or BPH. We evaluated the presence of pPSA in human serum by first using affinity purification to purify all forms of PSA present in a pool of human serum. We next fractionated the eluted PSA forms on HPLC and identified each PSA form based on its elution profile from the column. This analysis indicated that pPSA is present in human serum.
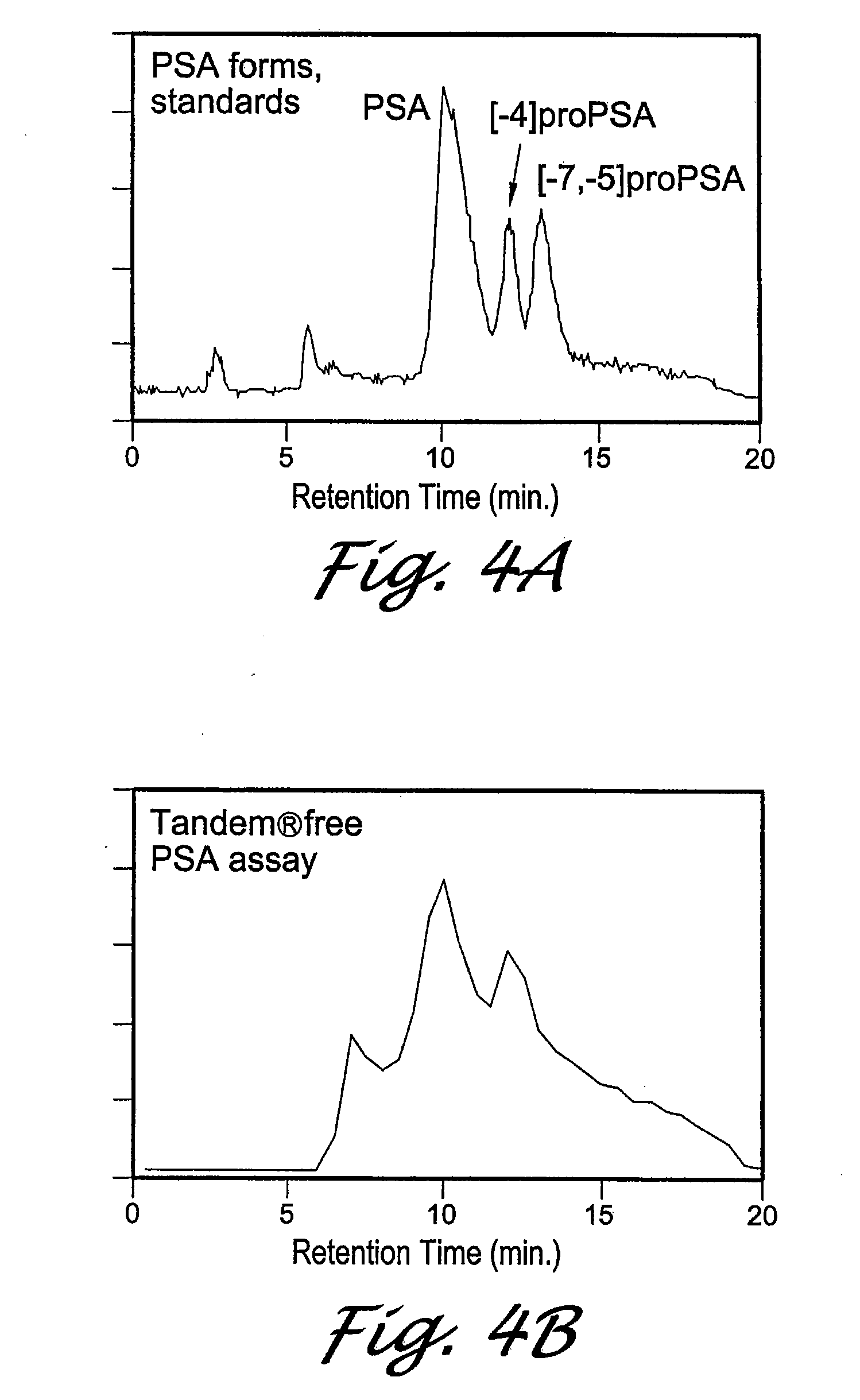
[0106]FIG. 4A shows the RT of standards of the different forms of PSA. All forms were verified by amino acid sequencing. The [−7,−5]pPSA peak contains approximately equal levels of [−7]pPSA and [−5]pPSA forms which are not resolved from each other.
[0107]FIG. 4B shows the profile of PSA forms from serum bound to the PSM7...
example 3
Detection of Truncated pPSA Forms in Prostate Cancer Serum
[0109]Serum from several individual men were also analyzed by Western blot using newly developed pPSA mAbs. PSA was purified from the serum of men who were biopsy-positive and biopsy-negative for prostate cancer. The serum PSA values of the 5 biopsy-positive men were 6, 9, 10, 18, and 24 ng / ml. Three biopsy-negative men with 7, 10, and 12 ng / ml total PSA were also analyzed. Since the free PSA represented only 10-20% of the total PSA, and it was not known what percentage of the free PSA might be comprised of pPSA forms, it was necessary to purify PSA from 100-200 mls of serum in order to be assured of adequate detection sensitivity for Western blot analysis. Total PSA was purified from the serum by immunoaffinity chromatography using the anti-PSA mAb, PSM773, which recognizes all forms of free PSA and PSA bound to ACT. The recovery of PSA ranged from 30-60% of the amounts calculated to be in the starting serum by immunoassay. ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| pH | aaaaa | aaaaa |
| pH | aaaaa | aaaaa |
| length | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com