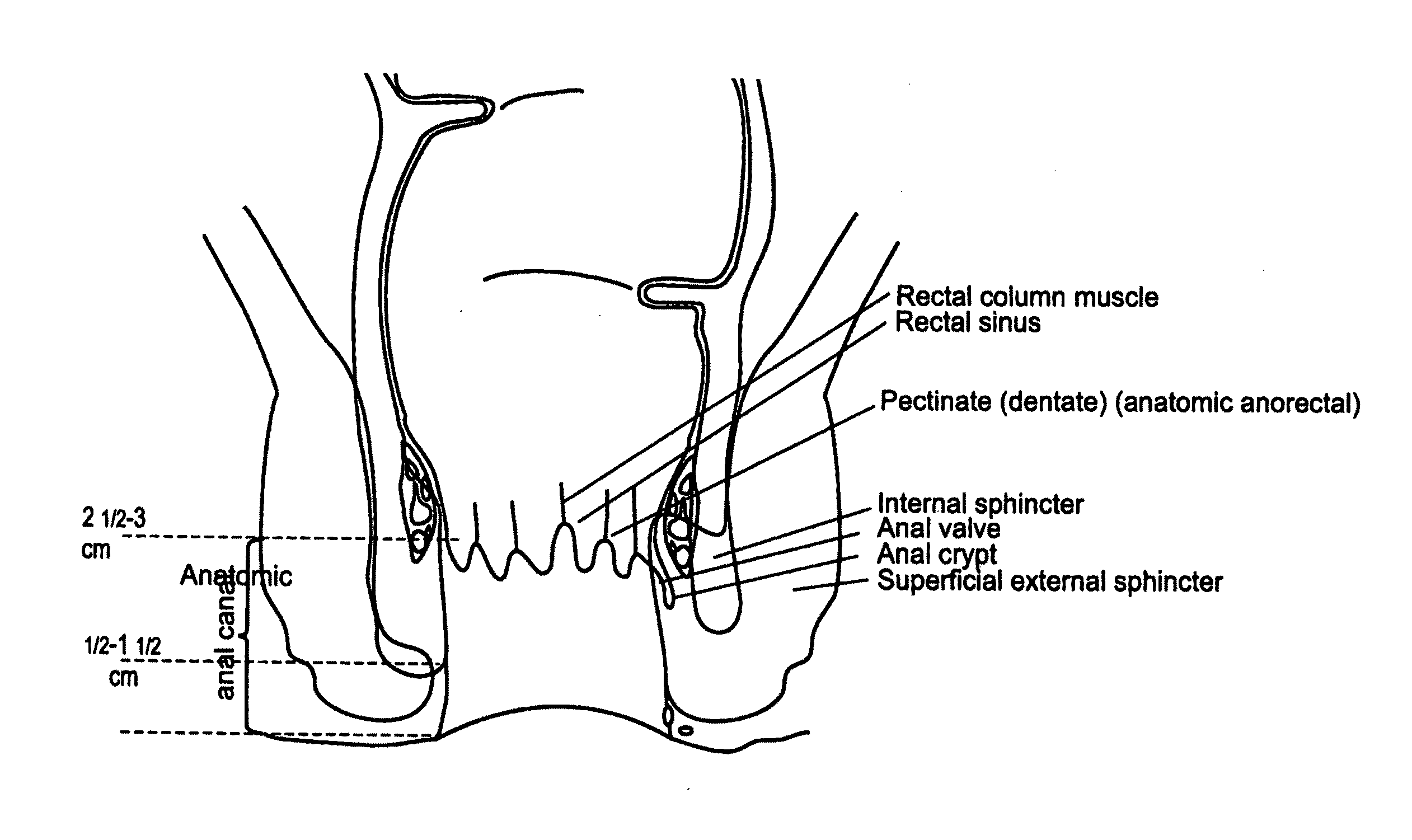
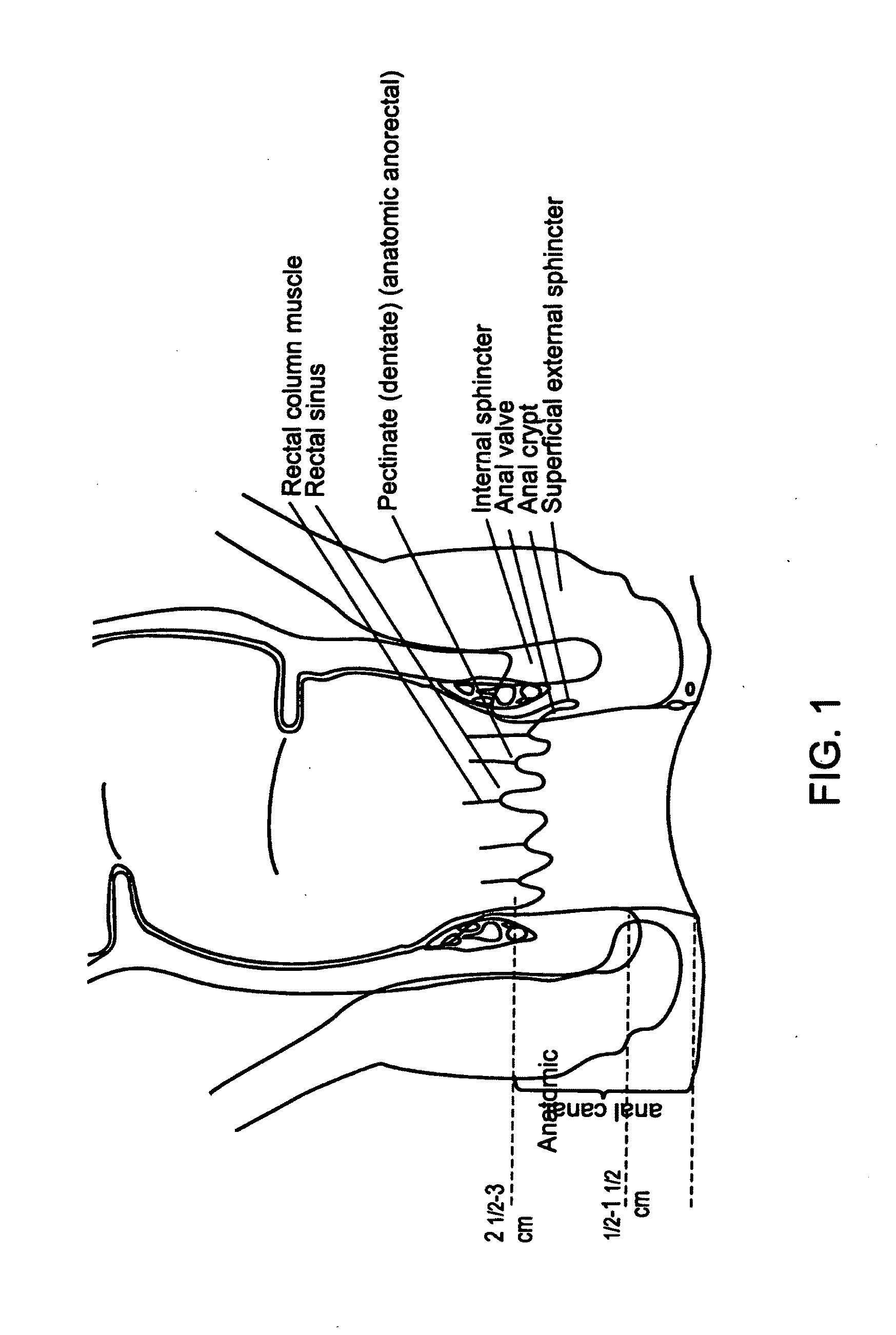
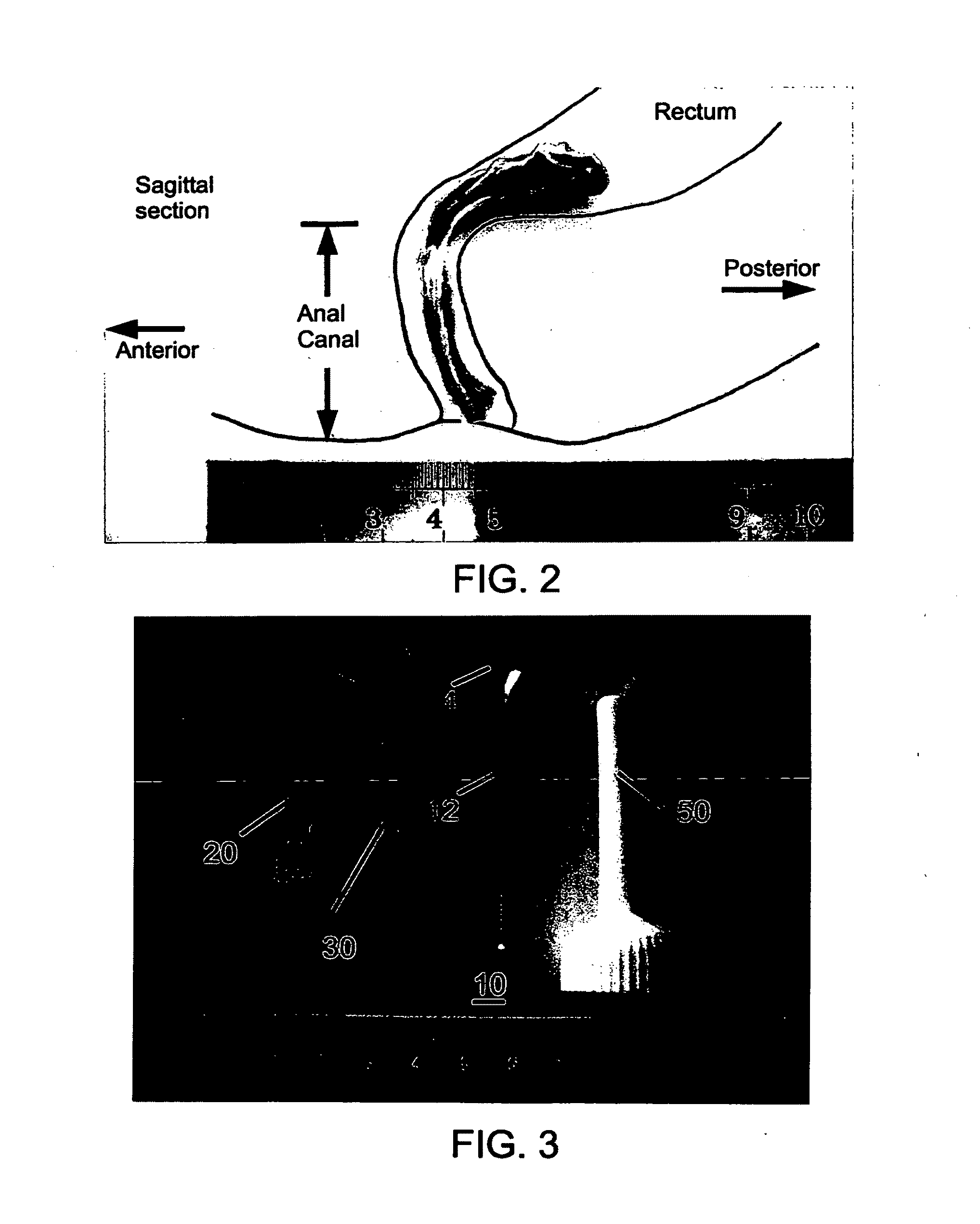
Fecal incontinence device, system and method
a technology of incontinence device and fecal incision, which is applied in the field of fecal incontinence device, system and method, can solve the problems of preventing many of them from being cared for at home, and the approach is not well tolerated by individuals
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
Preformed Silicone Plug
[0123]A silicone plug as shaped like plug 10 in FIG. 3 having a Shore A value of 3 was fabricated using well known silicone molding techniques. The plug was loaded into a hollow applicator and was self administered into the anal canal of a continent male subject (as shown in FIGS. 4A-C and 5A-C). The subject carried the plug for 24 hours. Following the 24 hour period, the plug was ejected with defecation and collected.
[0124]Trace fecal material present on the plug indicated that feces managed to creep under one side of the cap structure of the plug but was stopped at the neck region of the plug (just under the cap). The stem was free of fecal matter indicating that the tightest seal occurs on the stem just below the cap.
[0125]The subject reported that the plug did not induce any perceived discomfort and remained in position throughout the experiment. The subject also reported that flatulence escaped around the plug without inducing any resistance to gas releas...
example 2
Silicone Plug with Biasing Cap
[0126]The plug design similar to that shown in FIG. 7 made of silicone shore A 3 and inner sleeve insert made from silicone shore A 20 was tested on a female patient diagnosed with severe fecal incontinence (Wexner or Cleveland Clinic Fecal Incontinence scale of 20 out of 20). On a daily basis over the course of two weeks the plug was self administered by the subject into the anal canal (in the manner shown in FIGS. 8A-C). The subject carried each plug for ˜12-24 hours over which time she also wore incontinence pads in order to trap any leaked solids or liquids. Following each bowel movement with occurred roughly every 12-24 hours, the pads and the plug were collected and analyzed (see FIG. 10A-B). The pads showed no signs of soiling or leakage indicating that the plug effectively prevented involuntary loss of fecal matter and hence restored full continence to this subject. In addition, the top of the cap portion showed evidence of staining with fecal m...
example 3
Meltable Core Plug
[0127]The plug design similar to that shown in FIG. 9A-B with external shell 19 made of silicone shore A 40, filled through the bottom of stem portion 12 with molten Witespol™ solid fats that melts at 37 deg C., elongated while still molten by hanging a weight of 100 grams from it, allowed to cool and then sealed with a silicone RTV adhesive at the bottom of the stem portion 12 in order to keep the meltable core material fully contained in the external shell of plug 10. Plug 10 was tested on a female subject diagnosed with severe fecal incontinence (Wexner scale 20). On a daily basis over the course of a week the hollow plug was self administered by the subject into the anal canal in a manner similar to the insertion of a suppository. The subject carried each plug for ˜12-24 hours over which time she also wore incontinence pads in order to trap any leaked solids or liquids. Following each bowel movement with occurred roughly every 12-24 hours, the pads and the plug...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com