Anti-androgen peptides and uses thereof in cancer therapy
a technology of antiandrogen peptides and cancer therapy, which is applied in the direction of peptides, drug compositions, tissue cultures, etc., can solve the problems of tumors that are prostate cancer progresses to a hormone refractory state, and is unlikely to be completely resistant to androgens, so as to prevent the association of ar with src and remove the inhibitory action of sh3 domain
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
Preparation of AR-Derived Src-SH3 Binder Peptide (SEQ. ID. No. 1) by Chemical Synthesis
[0086]Peptides 1 through 4 can be conveniently manually prepared by applying the solid phase method (Bodansky M and Bodansky A, 1995) and the Fmoc / tBu (Fmoc: 9-fluorenyl-methoxycarbonyl) chemistry that is largely described in the scientific literature (Carpino and Han, 1972; Fields and Noble, 1990) and is well known to those skilled in the art. To expedite and facilitate the preparation, automatic multiple peptide synthesizers can be utilized. Any kind of chemical method or mechanic synthesizer with single or multiple channels can be also conveniently used to carry out the synthesis, without affecting the biological properties of the final compounds. The synthesis of the peptide is performed on a scale of 50 μmmoles using a resin suitably derivatized with a RINK linker capable to give C-terminal amide peptides (Rink, 1987). One of such resins is the product 4-(2′,4′-Dimethoxyphenyl-Fmoc-aminomethy...
example 2
“In Vitro” Anti Tumor Effect of the AR-Derived Src-SH3 Binder Peptide SEQ.ID NO.1
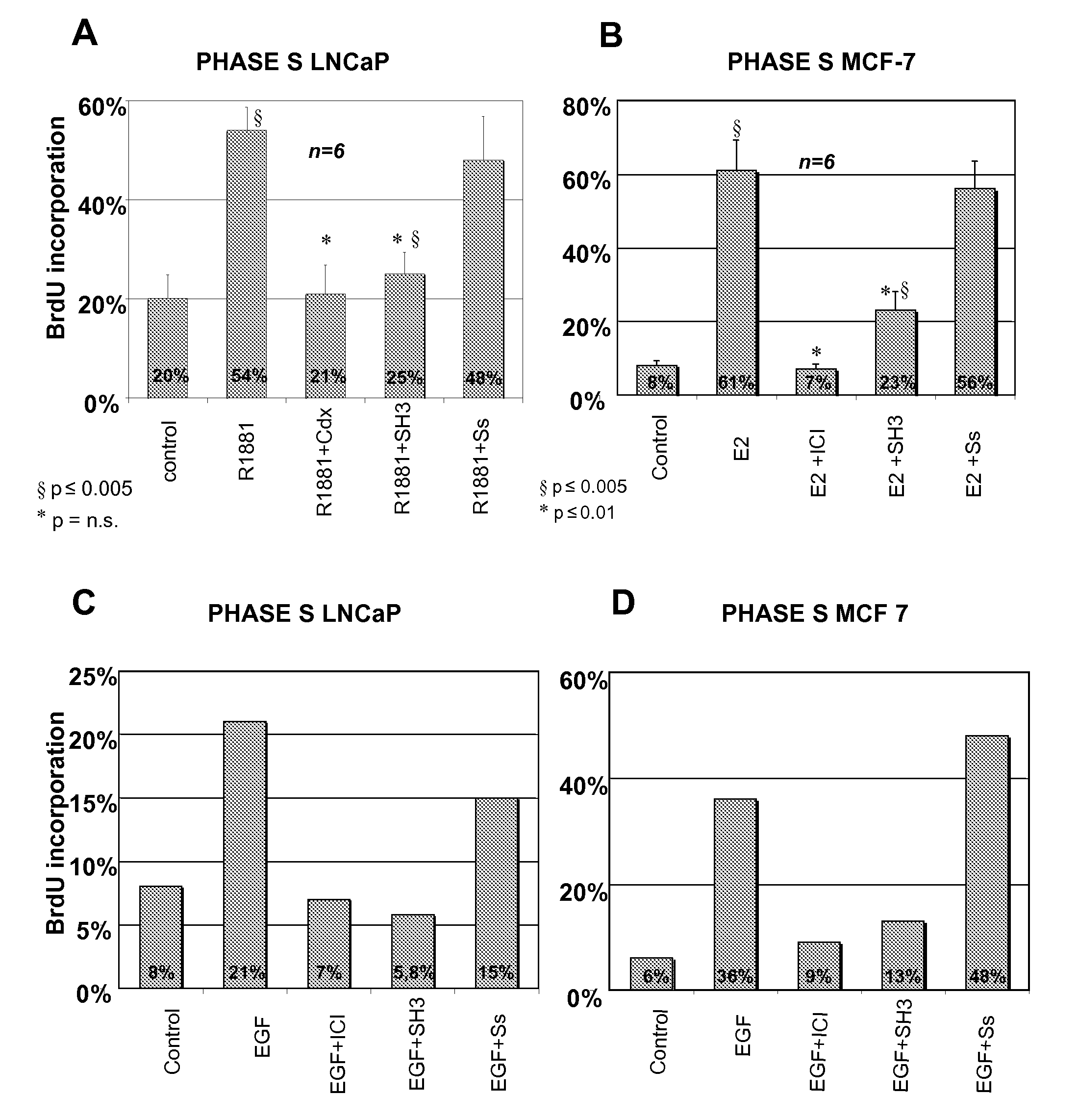
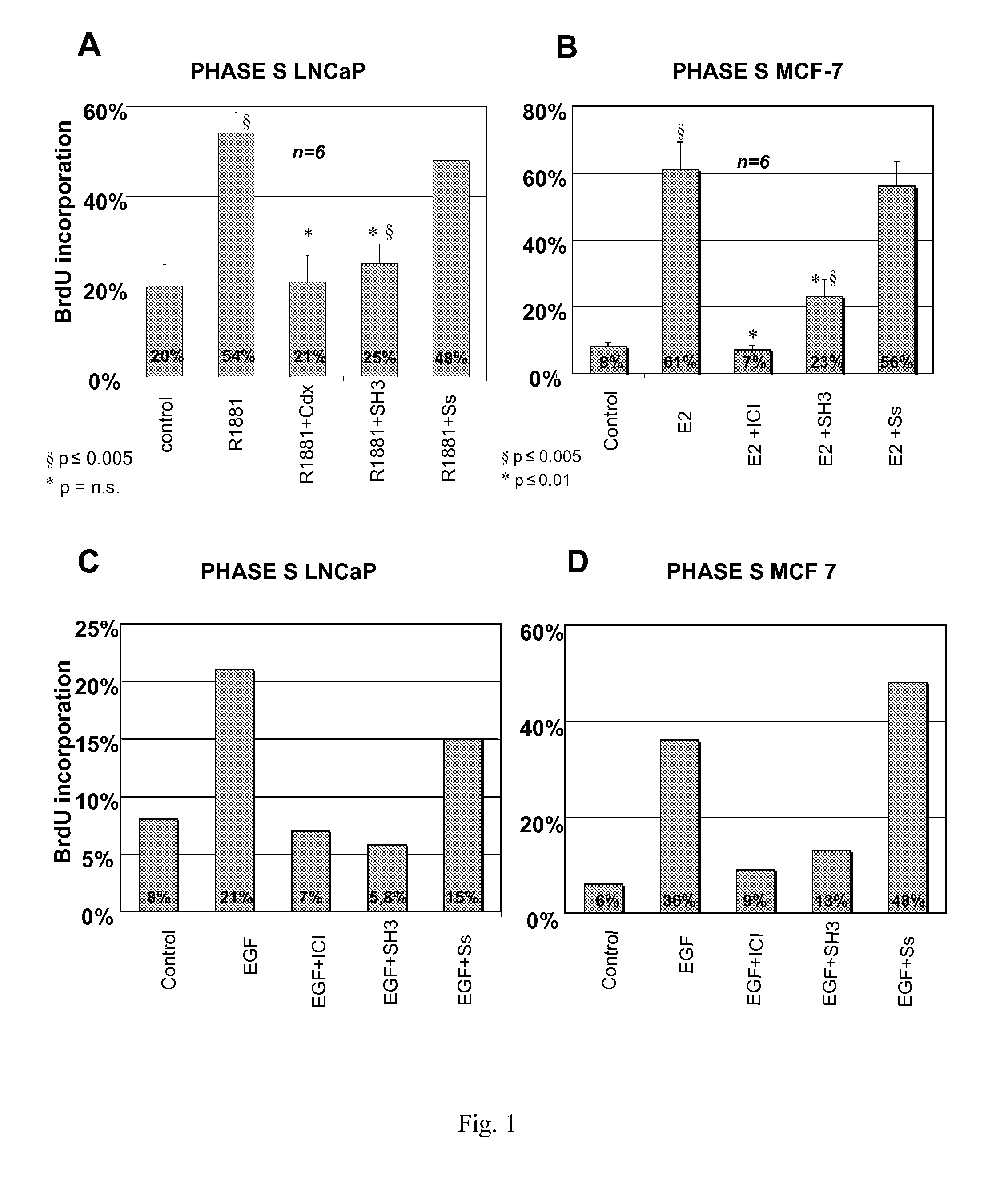
[0096]It has been shown that treatment of human prostate cancer LNCaP with 10 nM R1881 synthetic androgen strongly stimulates DNA synthesis. Therefore, LNCaP cells are routinely grown in 5% CO2 in air in RPMI 1640 medium (GIBCO) supplemented with phenol-red, 2 mM L-glutamine (GIBCO), penicillin (100 U / ml), gentamicin (50 μl / ml), insulin (Humulin I 0.2 U.I / ml, Roche) and 10% fetal calf serum. Cells are then seeded onto gelatine-precoated coverslips at about 40% confluence and maintained in phenol red-free RPMI-1640 containing insulin and charcoal stripped calf serum to assure minimal to no steroid contamination (Shi et al., 1994) for 3 days. LNCaP cells are then treated for 24 hrs with 10 nM of the synthetic androgen R1881 (Astra-Zeneca) alone or in the presence of a 1000-fold molar excess of the anti-androgen Casodex (Astra-Zeneca) or with the indicated peptides.
[0097]Results presented in FIG. 1A, avera...
example 3
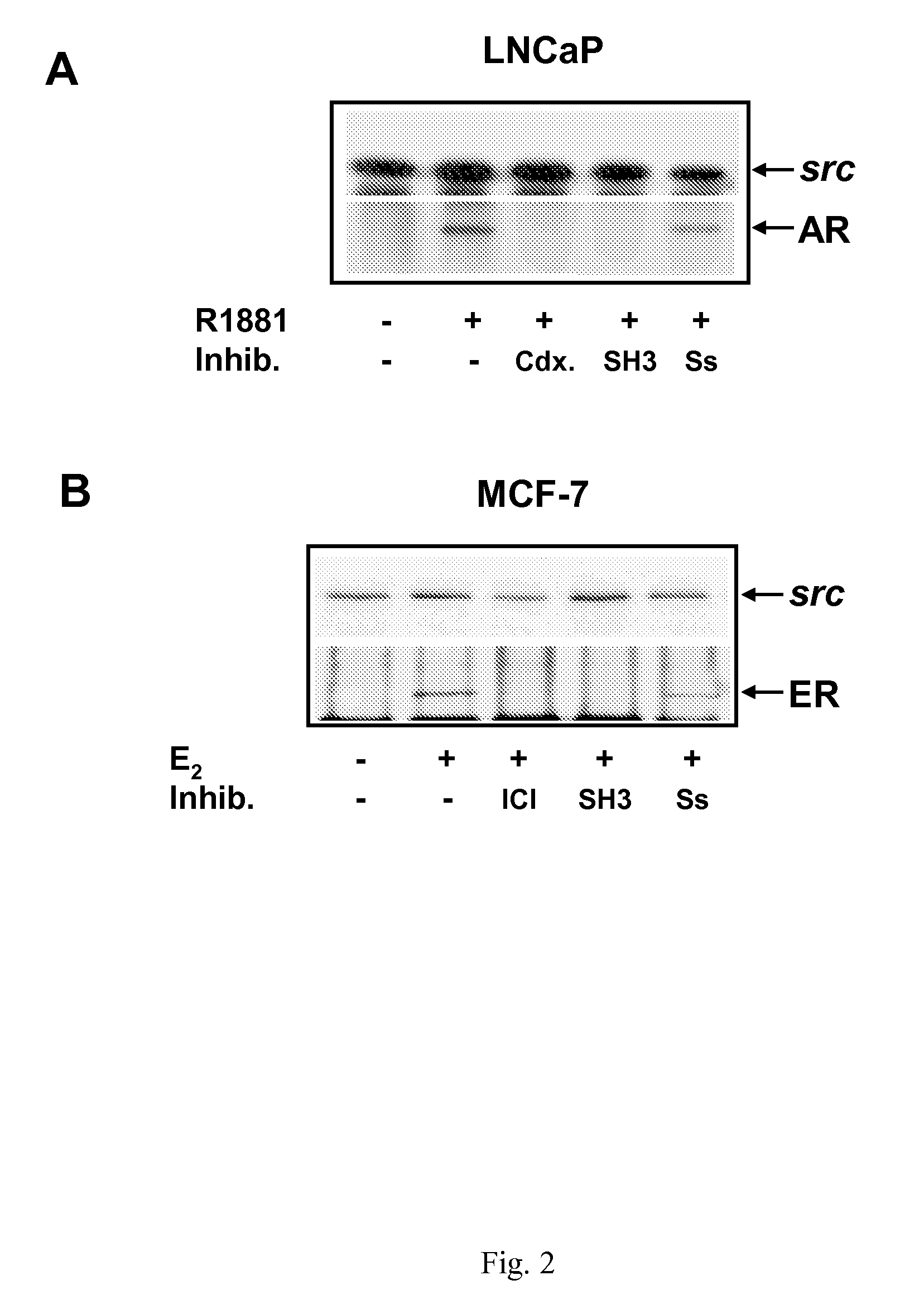
Inhibition of Androgen-Stimulated AR Association with Src by AR-Derived (Src-SH3 Binder) Peptide (SEQ.ID NO.1)
[0103]It has been demonstrated that hormone bound AR interacts with SH3 domain of Src kinase probably through a proline stretch (Migliaccio et al., 2000). As a consequence of this interaction, the kinase and the downstream signaling pathways are activated and, finally, DNA synthesis is activated. Use of a small peptide sequences mimicking the domain of AR involved in this interaction should be able to inhibit by competition the AR-Src association and block DNA synthesis. To test this hypothesis LNCaP cells are routinely grown in 5% CO2 in air in RPMI 1640 medium (GIBCO) supplemented with phenol-red, 2 mM L-glutamine (GIBCO), penicillin (100 U / ml), gentamicin (50 μl / ml), insulin (0.2 U.I. / ml) and 10% fetal calf serum. The cell are then kept in RPMI 1640 without phenol red and supplemented with glutamine, penicillin, gentamicin and insulin as above and containing 10% charcoal-...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Therapeutic | aaaaa | aaaaa |
| Nucleic acid sequence | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com