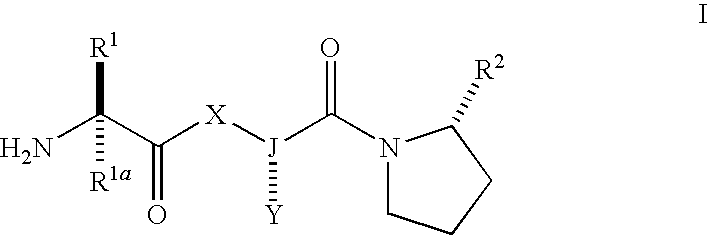
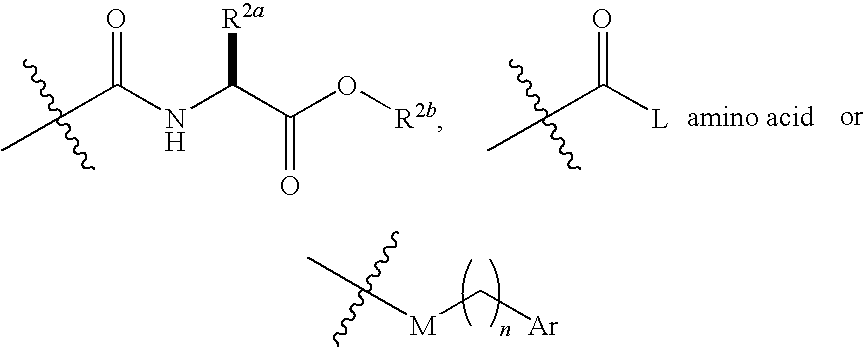
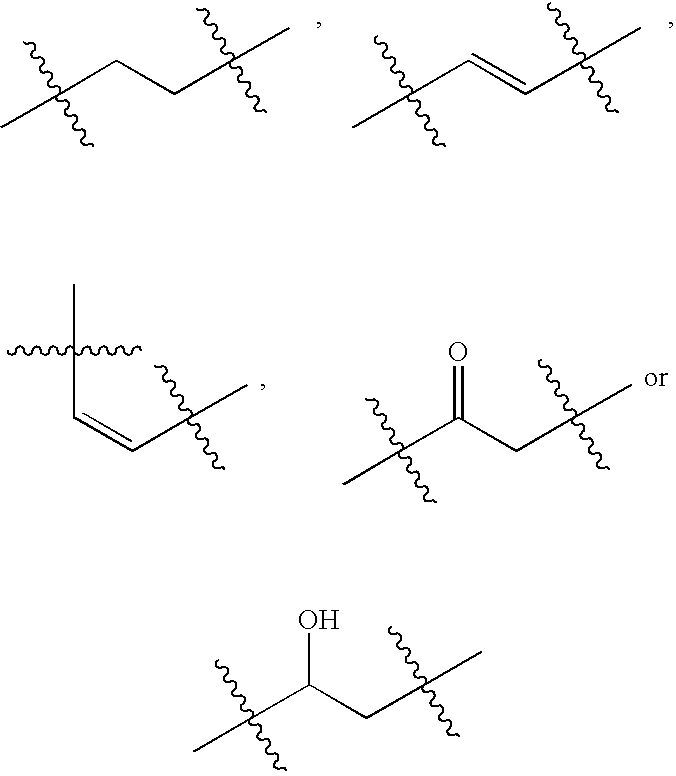
Iap binding compounds
a technology of iap and binding compounds, which is applied in the direction of instruments, peptides/protein ingredients, peptides, etc., can solve the problems of short half-life due to proteolytic degradation in the body, lack of molecular specificity of therapies, and inability to disclose or teach structural bases, etc., to achieve easy or less expensive synthesizing, the effect of improving the pharmacologic properties
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
Synthesis of Certain Compounds of the Invention and Components Thereof
[0142]
[0143](4-bromo-butyl)-benzene (50 a) (PAA 2-68). Phosphorus tribromide (1.10 mL, 11.2 mmol) was added dropwise to (4-hydroxy-butyl)-benzene (5.04 g, 33.5 mmol) and stirred 1 h under argon at 23° C. The flask was then heated to 100° C. via silicone oil bath and allowed to stir 4 h. The reaction mixture was cooled, quenched with several mL of cold H2O, diluted with ether, washed with brine (2×20 mL), dried over sodium sulfate, and concentrated in vacuo to 6.32 g (88%) of colorless oil. 1H NMR (CDCl3, 300 MHz) δ 7.18-7.33 (m, 5H), 3.44 (t, J=6.9 Hz, 2H), 2.66 (t, J=6.9 Hz, 2H), 1.76-1.96 (m, 4H); 13C NMR (CDCl3, 75 MHz) δ 147.8, 128.4, 125.8, 35.0, 33.7, 32.2, 29.8.
[0144](5-bromo-pentyl)-benzene (50 b) (PAA 67). Phosphorus tribromide (0.47 mL, 5.0 mmol) was added dropwise to (5-hydroxy-pentyl)-benzene (1.98 g, 12.0 mmol) and stirred 1 h under argon at 23° C. The flask was then heated to 100° C. via silicone oil...
example 2
Synthesis of Certain Oxazole Compounds
[0173]Synthetic scheme for oxazole compounds
[0174]I. Formation of Boc-Ala-Ser-OMe (3):
[0175]1 eq. of Boc-Ala-succinimide ester (2) was added to an ice-cold solution of DIPEA (2 eq.) and H-Ser-methyl ester hydrochloride (R=H) or H-Thr-methyl ester hydrochloride (R=CH3) (1) in dry THF under argon. The solution was allowed to warm to RT and stirred overnight (Rocchi, R. et al. (1987) Int. J. Peptide Protein Res. 30, 240-256). The THF was removed in vacuo, the resin was brought up in ethyl acetate and washed three times each with saturated sodium bicarbonate, 5% citric acid and saturated sodium chloride. The solution was dried with magnesium sulfate and the solvent was removed in vacuo leaving a white solid. Single spot on TLC (1:10 MeOH / CHCl3). No purification was necessary. 75% yield.
[0176]II. Formation of Boc-Ala-Ser-OMe Oxazoline (4):
[0177]1.05 eq. Burgess' reagent was added in one portion to a stirred solution of 3 in dry THF and the resulting ...
example 3
Synthesis of Compounds Containing Proline or Phenylalanine Derivatives
[0186]Materials. Unless otherwise stated, materials were purchased from Aldrich Chemical Co. (Milwaukee, Wis.) or Fisher Scientific (Pittsburgh, Pa.) and used without further purification. All unnatural phenylalanine derivatives were purchased from Advanced ChemTech (Louisville, Ky.) or Novabiochem (Calbiochem, San Diego, Calif.) as the Fmoc protected amino acid. These and the proline derivatives were used in peptide synthesis in the same manner as a naturally-occurring amino acid would be. Methylbenzhydrylamine (MBHA) solid-phase peptide synthesis resin, Rink amide resin, and 9-Fluorenylmethoxycarbonyl (Fmoc) protected amino acids were obtained from Advanced ChemTech (Louisville, Ky.) and NovaBiochem (San Diego, Calif.). 6-Bromoacetyl-2-dimethylaminonaphthalene (badan) dye was obtained from Molecular Probes (Eugene, Oreg.).
[0187]Peptide synthesis. Peptide molecules were synthesized on an Advanced ChemTech 396 MPS...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com