Genome-wide analysis of palindrome formation and DNA methylation
a gene and methylation technology, applied in the field of gene-wide analysis of palindrome formation and dna methylation, can solve the problems of limited examples, dna preparation, and inability to detect dsb repair,
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
example 1
[0063]The following example describes a process for genome-wide assessment of palindrome formation.
Methods
[0064]D79IR-8 and D79IR-8-Sce 2 cells were previously described (Tanaka et al., Proc. Natl. Acad. Sci. USA 99:8772-8777 (2002)). Colo320DM and RD were obtained from American Type Culture Collection. MCF7 and AG 1113215 were from the University of Washington. Skin biopsy derived fibroblasts HDF1 and HDF3 were obtained from the University of Washington and human foreskin fibroblasts HFF2 from the Fred Hutchinson Cancer Research Center (FHCRC) as anonymous cell lines. DNA samples stripped of identifying information from five primary medulloblastomas were provided by the Fred Hutchinson Cancer Research Center. All samples were obtained after Fred Hutchinson Cancer Research Center Institutional Review Board review and approval for use of anonymous human DNA samples and human cell lines.
Linkers and Oligonucleotides
[0065]Oligonucleotides were synthesized by...
example 2
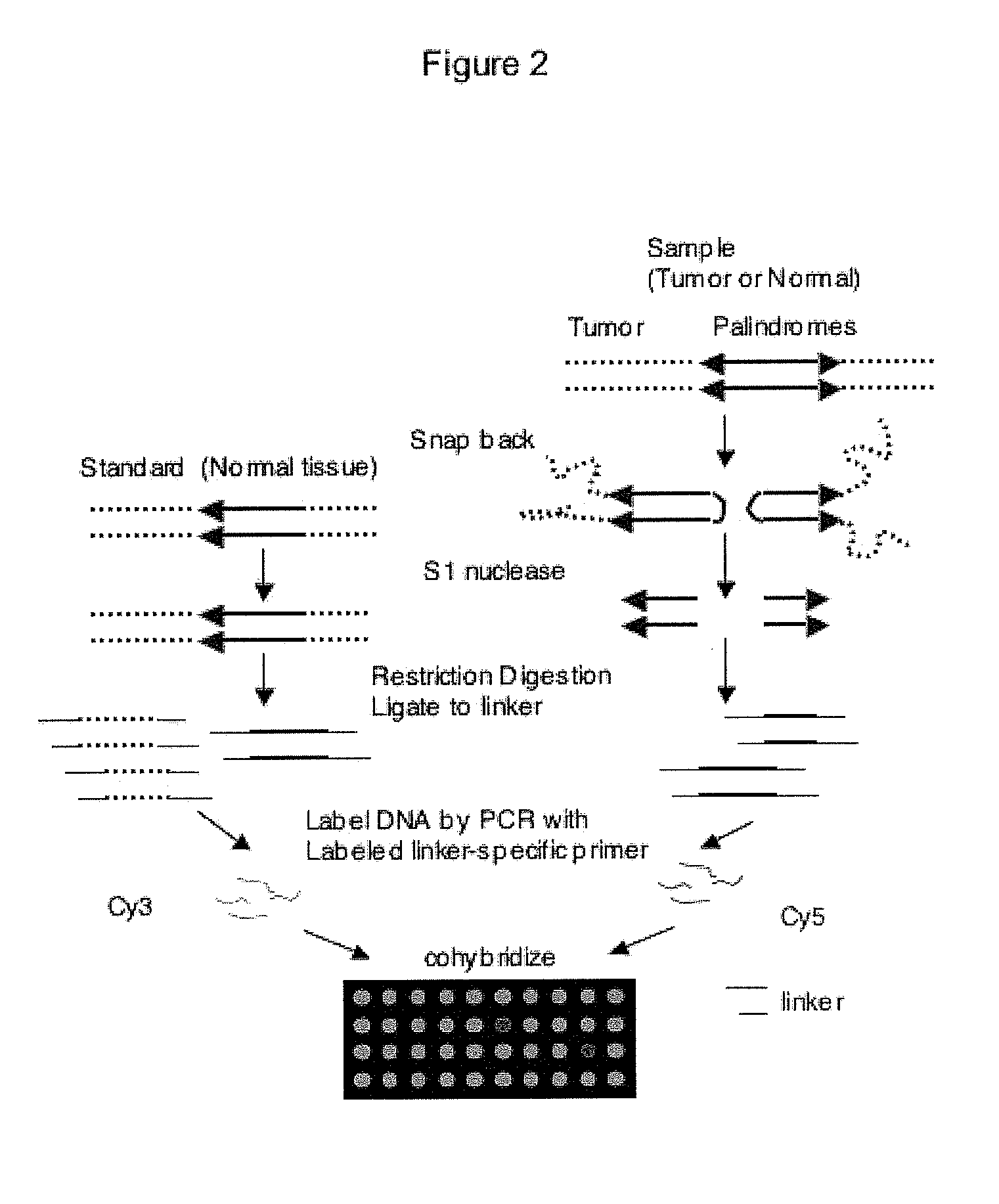
[0086]The following example demonstrates the use of ligation-mediated PCR to isolate a DNA fragment enriched in unmethylated CpG islands in a mammalian cell. A schematic of the process is provided as FIG. 8A.
[0087]Briefly, mouse genomic DNA was digested with a methylation sensitive restriction enzyme (for example, HpaII). The MspI linkers used above in Example 1 were used to ligate the HpaII fragments. The ligated DNA was amplified by PCR using the MspI primer from Example 1 (SEQ ID NO: 6). The method resulted in the specific amplification of HpaII digested genomic DNA of less than 500 base pairs (FIG. 8B). Random cloning and sequencing of the PCR products revealed that more than 50% of clones were at the CpG islands as defined using stringent criteria. (Takai and Jones, Proc. Natl. Acad. Sci. USA 99:3740-3745 (2002); incorporated herein by reference). In contrast, amplification of DNA digested with methylation-resistant isoschizomer MspI gave no clones near CpG islands.
TABLE 1Resul...
example 3
[0089]The following example describes methods used to identify palindromes and methylated DNA.
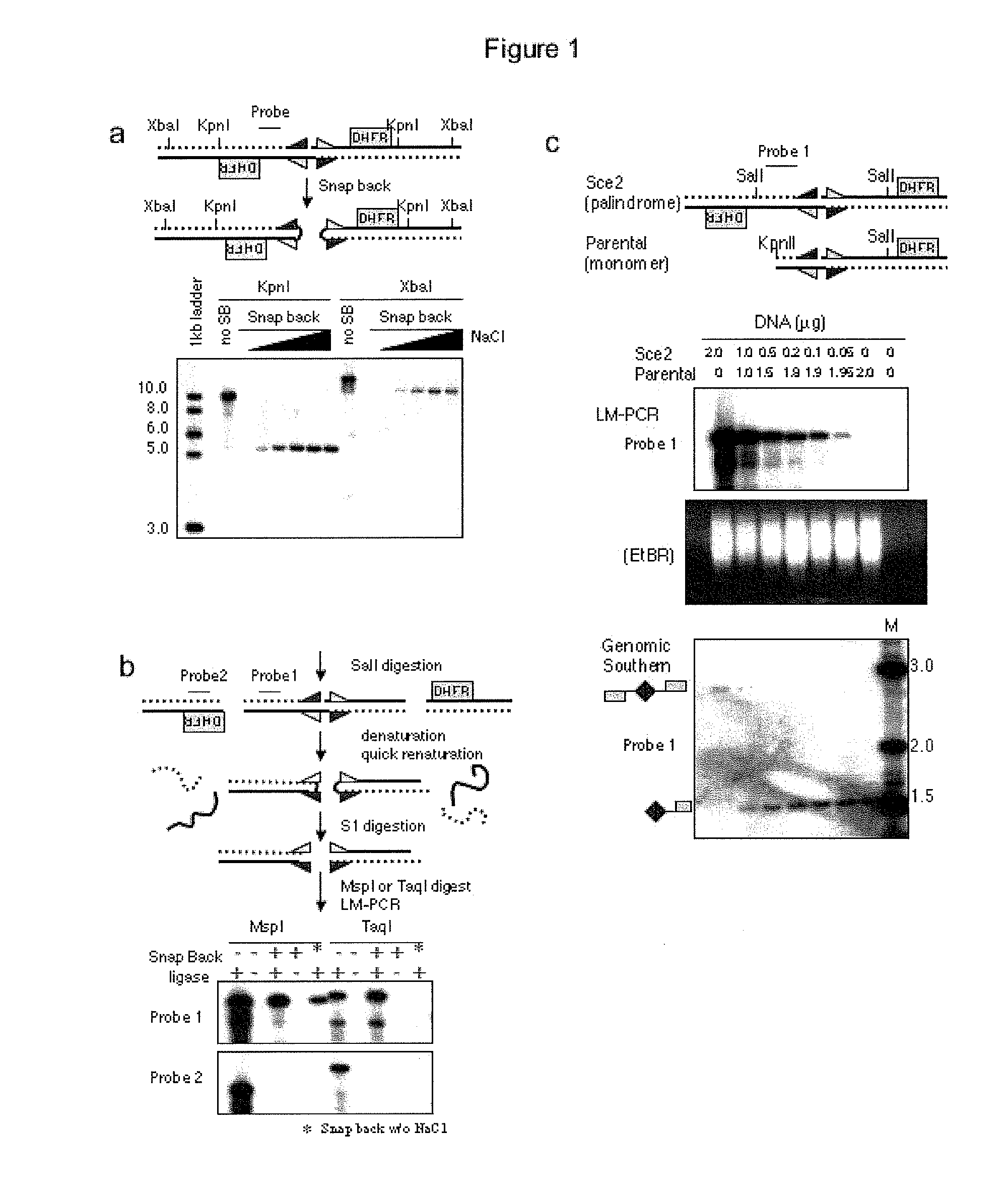
[0090]Above is described a method to obtain a genome-wide analysis of palindrome formation (GAPF) based on the efficient intrastrand base pairing in large palindromic sequences (Tanaka et al., Nat. Genet. 37:320-327 (2005)). Palindromic sequences can rapidly anneal intramolecularly to form ‘snap-back’ DNA under conditions that do not favor intermolecular annealing. This snap-back property was used to enrich for palindromic sequences in total genomic DNA by denaturing the DNA at 100° C. in the presence of 100 mM NaCl, rapidly renaturing it by snap cooling, and then digesting the mixture with a single-strand specific nuclease. Snap-back DNA formed from palindromes was double-stranded and resistant to the single-strand specific nuclease, whereas the remainder of genomic DNA was single-stranded and thus was sensitive to digestion (FIG. 9). Using this assay, de novo palindromes were shown to for...
PUM
| Property | Measurement | Unit |
|---|---|---|
| melting temperature | aaaaa | aaaaa |
| melting temperature | aaaaa | aaaaa |
| temperatures | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com