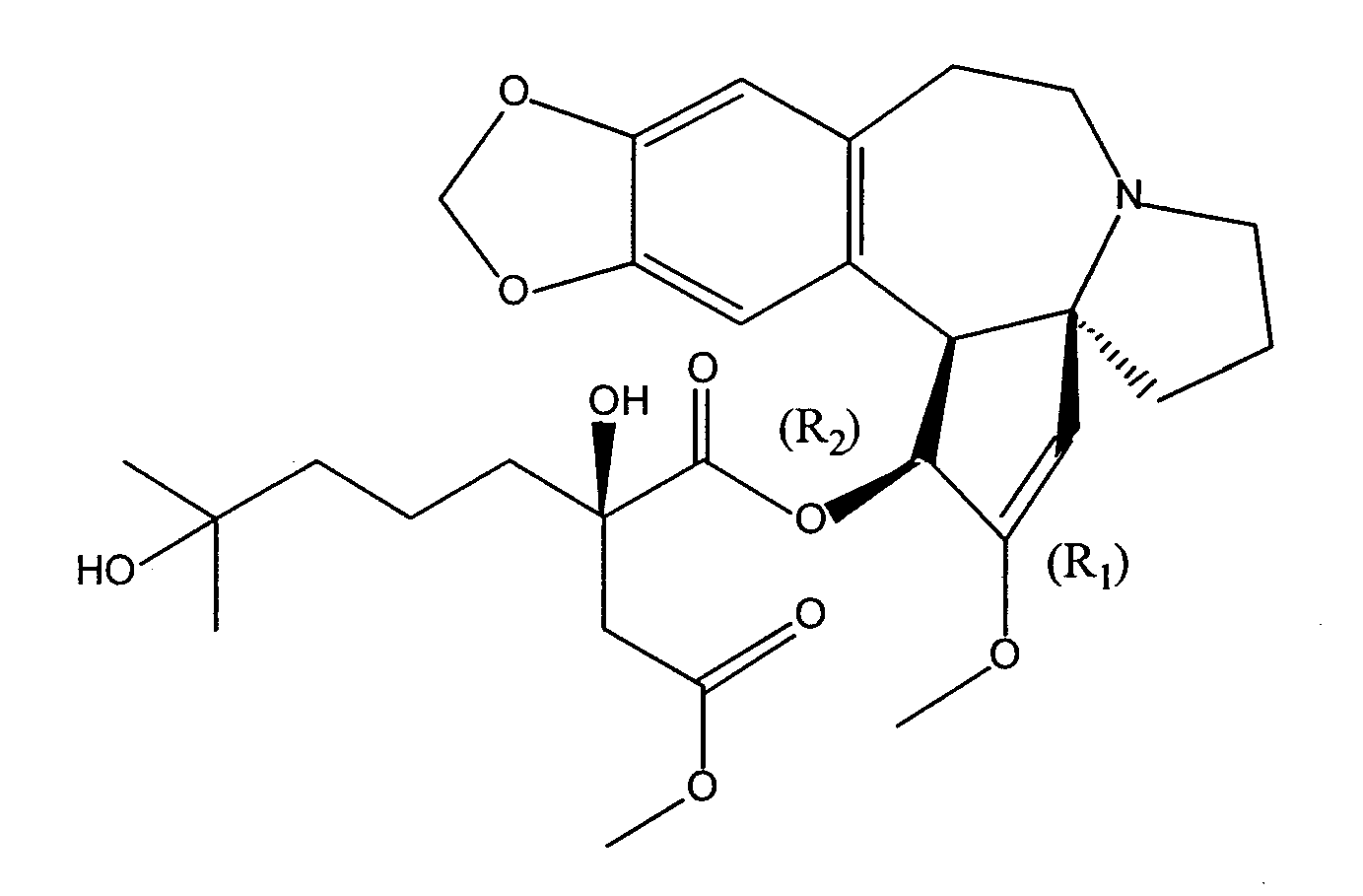
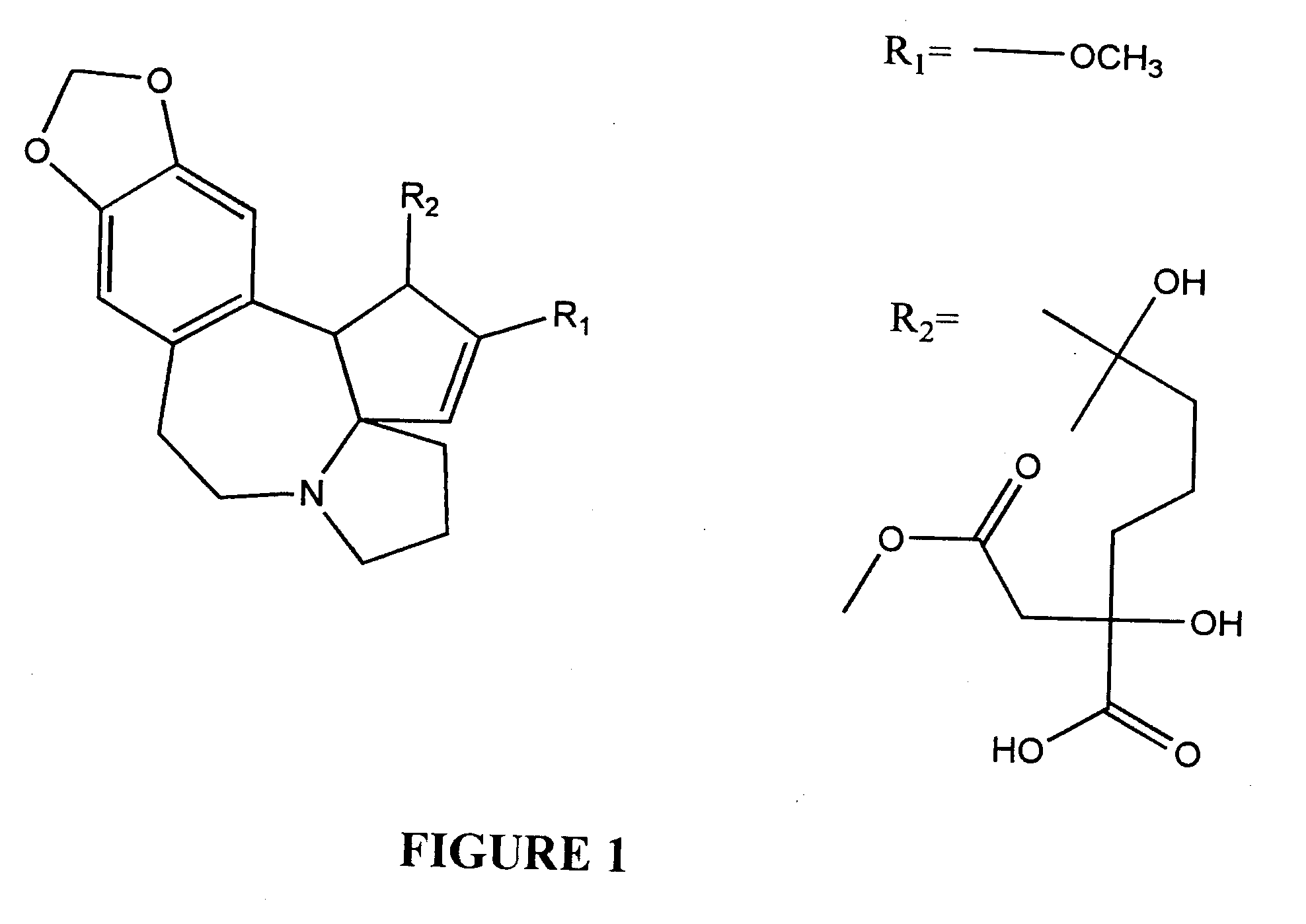
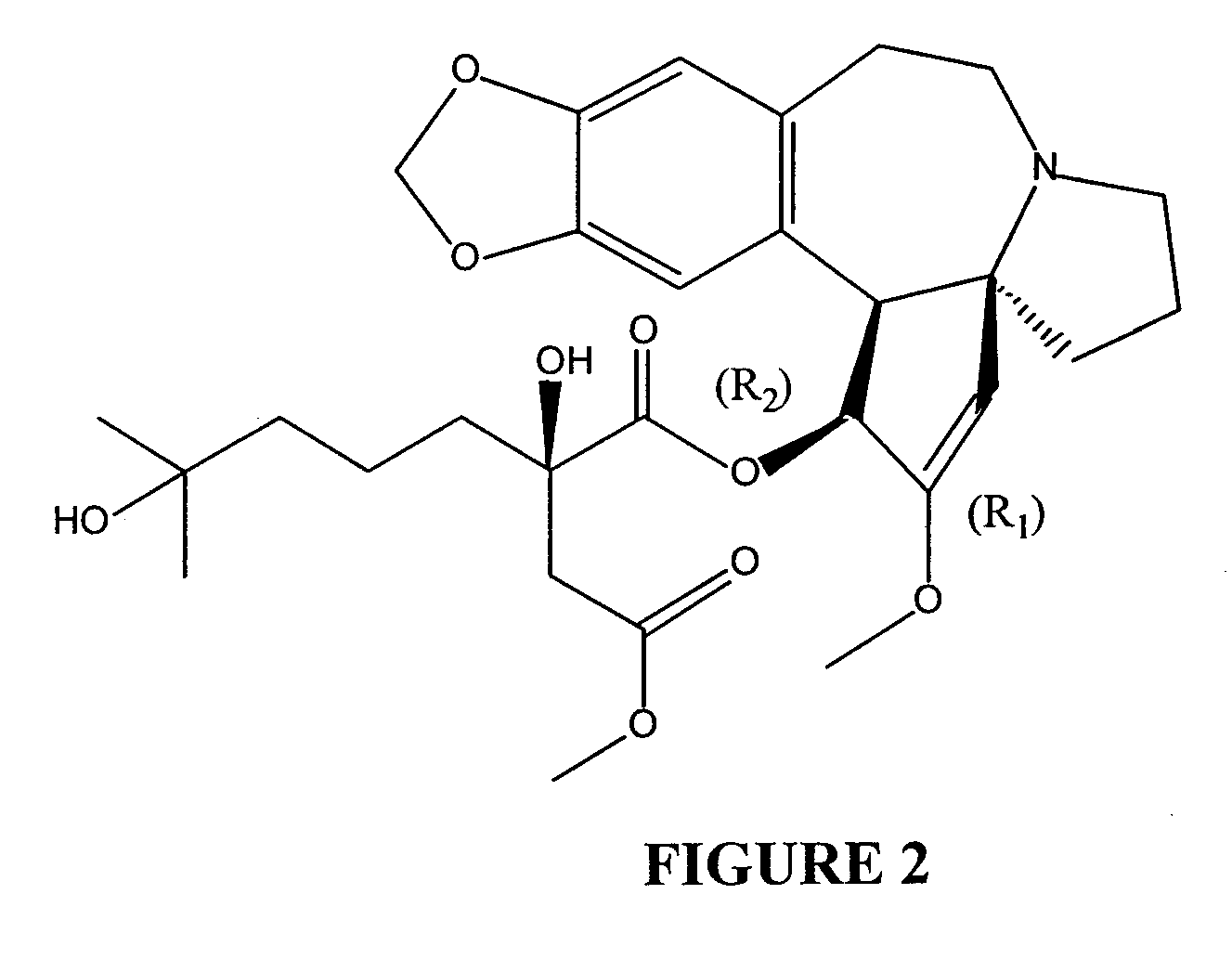
Cephalotaxine Alkaloid Compositions and Uses Thereof
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
example 1
Chemopotentiation of Cisplatin (CDDP) by Homoharringtonine (HHT)
[0020]Transplantable experimental murine fibrosarcomas (2×105 RIF-1 cells) were grown intradermally in the flanks of 3 month old female C3H mice (Charles River, Holister, Calif.). When the tumors reached a volume of approximately 100 mm3, the mice were randomly assigned to each experimental group (4 mice per group).
[0021]The experimental compositions were prepared as described in Table 2.
TABLE 2AgentDoseSolventSupplierHomoharringtonine2 mg / kgDMSONCICisplatin4 mg / kgWater for injectionDavid Bull Labs
[0022]The chemopotentiator, homoharringtonine, was obtained from NCI and was made to the appropriate concentration in DMSO. Cisplatin (David Bull Laboratories-Mulgrave, Australia, lot. 5201844x) was made to the appropriate concentration in water for injection. The compositions were injected systemically (i.e., intraperitoneally, i.p.), in a volume of 100 microliters. For the treatment of group 3, the chemopotentiator, homoharr...
example 2
Effect of Homoharringtonine, Alone and in Combination with Other Chemotherapeutics on RIF-1 Tumor Growth in C3H Mice
[0026]The RIF-1 murine fibrosarcoma tumor model was used to evaluate the antitumor activity of homoharringtonine, alone and in combination with various antiproliferative agents. The antiproliferative agents used include those that affect nucleic acid (e.g., DNA) integrity (e.g., cisplatin, cytarabine, camptothecin, etoposide, 5-fluorouracil, or amonafide), agents that affect structural proteins (e.g., paclitaxel, vinblastine, or colchicine) or cytoplasmic enzymes (e.g., genistein).
[0027]Homoharringtonine (HHT-NCI) was obtained from NCI as a powder. Homoharringtonine (HHT-Clin) was obtained from Hangzhou Minsheng Pharmaceutical Group (Hangzhou, China), in 1 mL vials, prediluted with water to 1 mg / mL. Cisplatin for Injection, USP, was obtained from David Bull Labs (Mulgrave, Australia), Lot No. 5201844x, as a lypholized powder. Paclitaxel was obtained from Bristol Myers ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Volume | aaaaa | aaaaa |
| Fraction | aaaaa | aaaaa |
| Fraction | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com