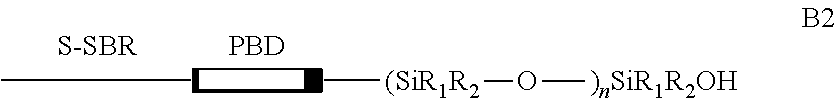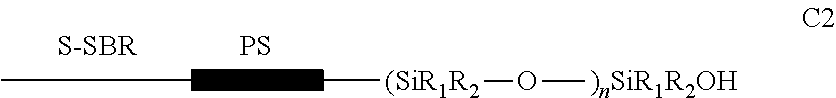Process for the Preparation of a 1,3-Butadiene and Styrene Copolymer Containing a Random Section in its Main Chain Followed by a Block with a Structure Differentiated from the Main Chain, Homopolymeric or Copolymeric, Functionalized and the Product Obtained From This
a technology of 1,3-butadiene and styrene, which is applied in the field of process for the preparation of a 1,3-butadiene and styrene copolymer containing a random section in its main chain followed by a block with a structure differentiated from the main chain, homopolymeric or copolymer, functionalized and the product obtained from this. it can solve the problems of reducing the performance of one of the others and being impossibl
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1 (
B2)
[0104]Preparation of an elastomer of type s-SBR functionalized, whose main polymeric chains have a random distribution in their constituent mers and a controlled microstructure, containing an end block of polybutadiene, followed by a continuous sequence of the siloxane functional group (—[—Si(CH3)2—O—]—) and a silanol termination (—Si(CH3)2—OH).
[0105]A schematic representation of the structure of this elastomer is presented below:
where, R1 and R2═CH3; PBD=block of polybutadiene; n=n° of siloxane units
[0106]In a 2 liter capacity reactor, equipped with a turbine type mechanical agitator and a refrigeration cover, the first step was performed of the anionic copolymerization of the 1,3-butadiene monomers and styrene, in a solution of cyclohexane, with the polar additive TMEDA, and using n-butyl-lithium as the initiator.
[0107]For this copolymerization, the reactor was filled with 182 g of 1,3-butadiene, 46 g of styrene, 1290 g of cyclohexane and 1.5 g of TMEDA, aiming for a chemical c...
example 2 (
C2)
[0121]Preparation of an elastomer of type s-SBR functionalized, whose main polymeric chains have random distribution in their constituent mers and a controlled microstructure, containing an end block of polystyrene, followed by a continuous sequence of the siloxane functional group (—[—Si(CH3)2—O—]—) and a silanol termination (—Si(CH3)2—OH).
[0122]A schematic representation of the structure of this elastomer is presented below:
where, R1 and R2═CH3; PS=block of polystyrene; n=n° of siloxane units
[0123]In a 2 liter capacity reactor, equipped with a turbine type mechanical agitator and a refrigeration cover, the first step was performed of the anionic copolymerization of the 1,3-butadiene monomers and styrene, in a solution of cyclohexane, with the polar additive TMEDA, and using n-butyl-lithium as the initiator.
[0124]For this copolymerization, the reactor was filled with 146 g of 1,3-butadiene, 37 g of styrene, 1343 g of cyclohexane and 1.1 g of TMEDA.
[0125]For the initiation, a qua...
example 3
[0151]Vulcanized elastomeric compounds prepared with the group 1 elastomers.
[0152]The group 1 elastomers are type S-SBR. Their main polymeric chains have a random distribution in their constituent mers and a controlled microstructure, containing an end block of polybutadiene, followed by a continuous sequence of the siloxane functional group (—[—Si(CH3)2—O—]—) and a silanol termination (—Si(CH3)2—OH).
[0153]In Table 5, the main characteristics of these elastomers are presented.
TABLE 5S-SBRBlock ofTerminalElastomercompositionpolybutadieneFunctionalizationA221.0% styrene;not presentsiloxane groups and(reference)63.0% 1,2-terminal silanolvinyl (a)B119.0% styrene;Block with ansiloxane with an average64.1% 1,2-average of 50of 8.0 groups per chainvinyl (a)mers ofincluding silanol terminalbutadienegroupper chainB219.6% styrene;Block with ansiloxane with an average63.7% 1,2-average of 150of 5.0 groups per chainvinyl (a)mers ofincluding silanol terminalbutadienegroupper chain(a) = based on th...
PUM
| Property | Measurement | Unit |
|---|---|---|
| temperature | aaaaa | aaaaa |
| temperature | aaaaa | aaaaa |
| temperature | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com