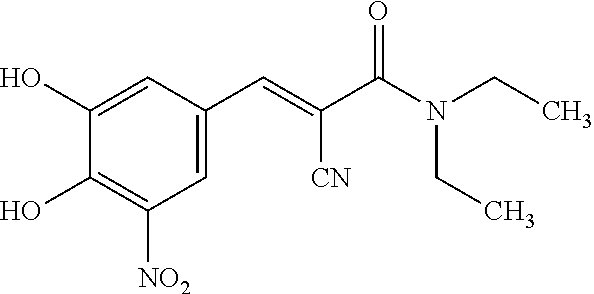
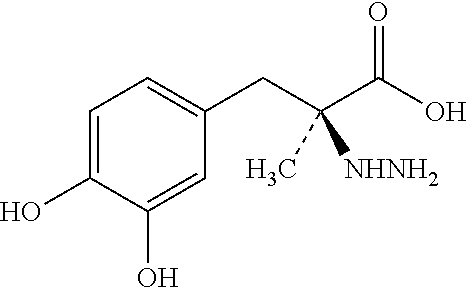
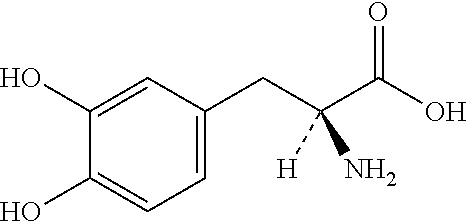
Single Unit Oral Dose Pharmaceutical Composition Comprising Levodopa, Carbidopa And Entacapone Or Salts Thereof
a single-unit, oral-dosage technology, applied in the direction of drug compositions, biocide, nervous disorders, etc., can solve the problems of side effects, other unwanted side effects, and regimens that are not patient-compliant, and achieve the effect of reducing the phenomenon of “wearing off”
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
examples
[0045]The composition of batches is provided in Table 1 to 23. Following formulations are representatives of the preferred compositions of the invention. The preparation of example is detailed below.
example-i
[0046]
TABLE 1Pharmaceutical composition of the inventionIngredients% w / wEntacapone IR layer / PortionEntacapone38.41Mannitol6.73Sodium starch glycolate3.85Corn Starch9.42Croscarmellose sodium4.62Povidone1.51Purified waterq.s.Extragranular IngredientsMagnesium stearate0.77Levodopa and Carbidopa CR layer / PortionLevodopa19.22Carbidopa5.19Microcrystalline Cellulose4.62Povidone3.46Purified Waterq.s.Extragranular IngredientsPoly Vinyl Pyrrolidone1.73Magnesium Stearate0.38
Procedure:
Entacapone Layer / Portion:
[0047]Entacapone and mannitol were co-milled and sifted. Cornstarch, croscarmellose sodium and sodium starch glycolate were co-sifted separately. The materials were placed in granulator and mixed. Povidone was dissolved in purified water and granulated with the mixed material. The granulated contents were dried. Magnesium stearate was sifted and mixed with the dried granules.
Levodopa and Carbidopa Layer / Portion:
[0048]Levodopa, Carbidopa and microcrystalline cellulose were co-sifted and mix...
example-ii
[0051]
TABLE 3Pharmaceutical composition of the inventionIngredients% w / wEntacapone layer / PortionEntacapone28.57Mannitol5.00Sodium starch glycolate2.86Corn Starch7.00Croscarmellose sodium3.43Povidone1.14Purified waterq.s.Extragranular IngredientsMagnesium stearate0.57Levodopa and Carbidopa CR layer / PortionLevodopa28.57Carbidopa7.71Microcrystalline Cellulose6.86Povidone5.14Purified Waterq.s.Extragranular IngredientsPVP2.57Magnesium Stearate0.57
Procedure:
Entacapone Layer / Portion:
[0052]Entacapone and mannitol were co-milled and sifted. Corn starch, croscarmellose sodium and sodium starch glycolate were co-sifted separately. The materials were placed in granulator and mixed. Povidone was dissolved in purified water and granulated with the mixed material. The granulated contents were dried. Magnesium stearate was sifted and mixed with the dried granules.
Levodopa and Carbidopa Layer / Portion:
[0053]Levodopa, Carbidopa and microcrystalline cellulose were co-sifted and mixed. Povidone was diss...
PUM
| Property | Measurement | Unit |
|---|---|---|
| time | aaaaa | aaaaa |
| pH | aaaaa | aaaaa |
| compositions | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com