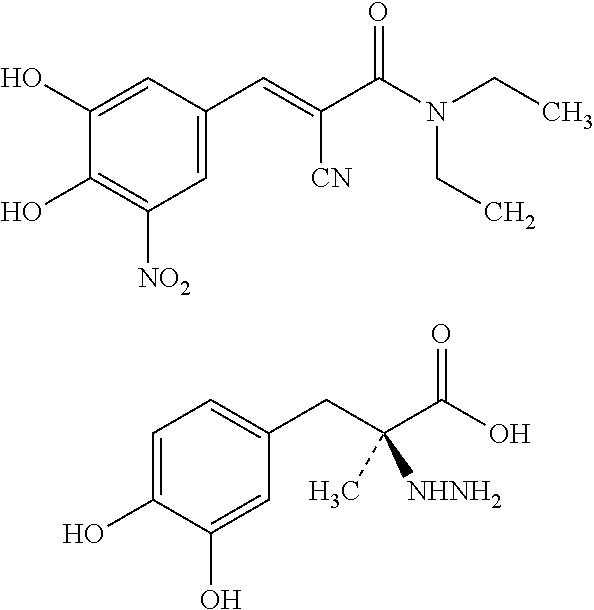
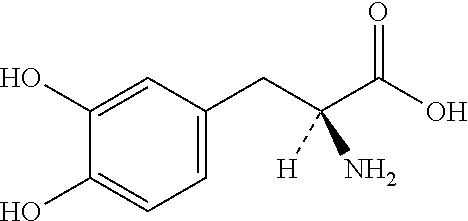
Extended Release Pharmaceutical Composition Of Entacapone Or Salts Thereof
a technology of entacapone and pharmaceutical composition, which is applied in the direction of drug compositions, biocide, nervous disorders, etc., can solve the problems of side effects, non-compliance with the patient's regimen, and other unwanted side effects, so as to reduce the ‘wearing off’ phenomenon. , the effect of reducing the ‘wearing off’ phenomenon
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
examples
[0061]The composition of batches is provided in Table 1 to 23. Following formulations are representatives of the preferred compositions of the invention. The preparation of example is detailed below.
example — i
Example—I
[0062]
TABLE 1Pharmaceutical composition of the invention% w / wIngredientsEntacapone60.1Mannitol18.0Hydroxypropyl methyl cellulose16.2Povidone0.9Magnesium Stearate0.9Talc0.2-3CoatingOpadry3.8
[0063]Procedure:
[0064]Entacapone and mannitol were sifted and co-sifted through mesh to form a bulk. Hydroxypropyl methyl cellulose was sifted and added to the above bulk to form a mixture. A part of magnesium stearate was sifted and mixed with the above mixture to form a bulk. The bulk was dry granulated using roller compactor to form the slugs. The slugs were size reduced to form granules. The granules were lubricated with remaining portion of magnesium stearate and talc to form a blend. The blend was compressed using suitable tooling to form tablets. The tablets were coated using Opadry.
TABLE 2Dissolution data of composition prepared as per example I.Time (hrs)% drug released0.528152274388496
[0065]Table 2 provides the dissolution data of composition prepared as per formula given in tab...
example — ii
Example—II
[0066]
TABLE 3Pharmaceutical composition of the inventionIngredients% w / wEntacapone57.1Lactose19.3Hydroxy ethyl cellulose12.4Hydroxypropyl methyl cellulose8.6Waterq.s.Magnesium Stearate1.4Talc1.1
[0067]Procedure: Entacapone, lactose, hydroxy ethyl cellulose and hydroxypropyl methylcellulose were co-sifted and mixed to form a bulk. The bulk was wet granulated using water to form the granules and the granules were dried. The dried granules were size reduced. Magnesium stearate and talc were sifted and added to the size reduced granules and were compressed using suitable tooling to form tablets.
TABLE 4Dissolution data of composition prepared as per example II.Time (hrs)% drug released0.533158278388497
[0068]Table 4 provides the dissolution data of composition prepared as per formula given in table 3. For determination of drug release rate, USP Type 2 Apparatus (rpm 75) was used wherein 1000 ml of pH 5.5 phosphate buffer at 37° C.±0.5° C. was used as medium.
PUM
| Property | Measurement | Unit |
|---|---|---|
| pH | aaaaa | aaaaa |
| time | aaaaa | aaaaa |
| hydrophilic | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com