Compound and method for regulating plasminogen activation and cell migration
a technology of plasminogen activation and cell migration, applied in the field of new drugs, can solve the problems of limiting efficiency and the war against heart disease and stroke, and achieve the effect of preventing cell migration and reducing capillary tube formation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example i
Transcytosis of p97 through BBCEC Monolayers
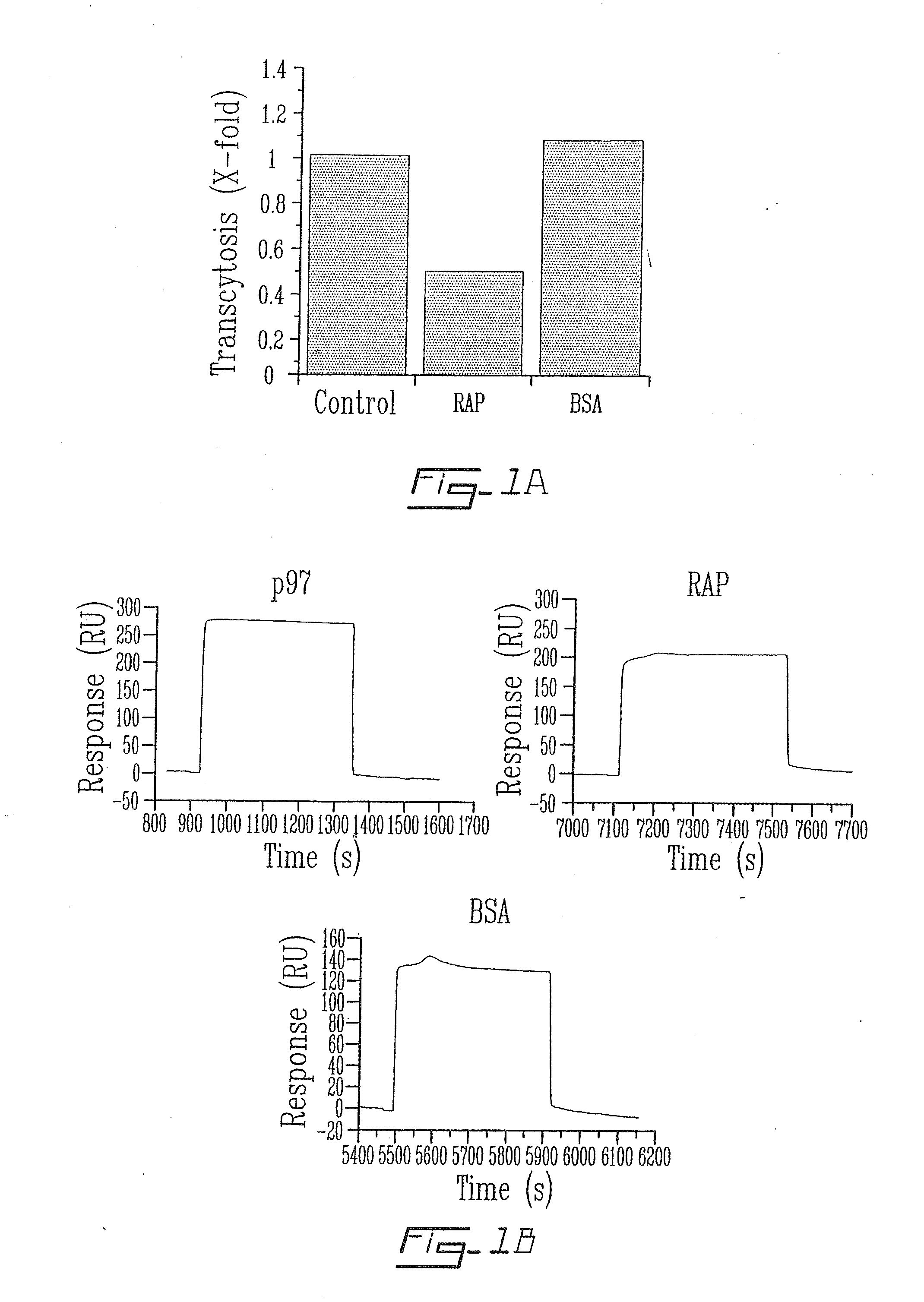
[0097]Transcytosis experiments are performed at 37° C. for 2 hrs. [125I]-p97 (25 nM) is added to the upper side of the cell-covered filter in the absence or presence of RAP (650 nM) or BSA (5 μM). At the end of the experiment, radiolabelled proteins are measured in the lower chamber of each well by TCA precipitation. Results represent means±SE (n=6) (FIG. 1A). In the second part of the experiment (FIG. 1B), p97 is immobilized on a sensor chip surface (CM5) as described in the Materials and Methods section above and p97, RAP and BSA (5 μg / 100 μl) are injected over the immobilized p97.
[0098]The first evaluation was the transcytosis of p97 across an in vitro model of the BBB at 37° C. (FIG. 1A). A significant (>50%) reduction in the transport of [125I]-p97 (25 nM) from the apical (blood side) to the basolateral side (brain side) of BBCEC monolayers was observed in the presence of 640 nM RAP. Transcytosis of [1251]-p97 was unaffected by a 200-...
example ii
Pro-uPA and p97 Interaction
[0099]Biospecific Interaction Analysis between p97 and anti-p97 mAbs
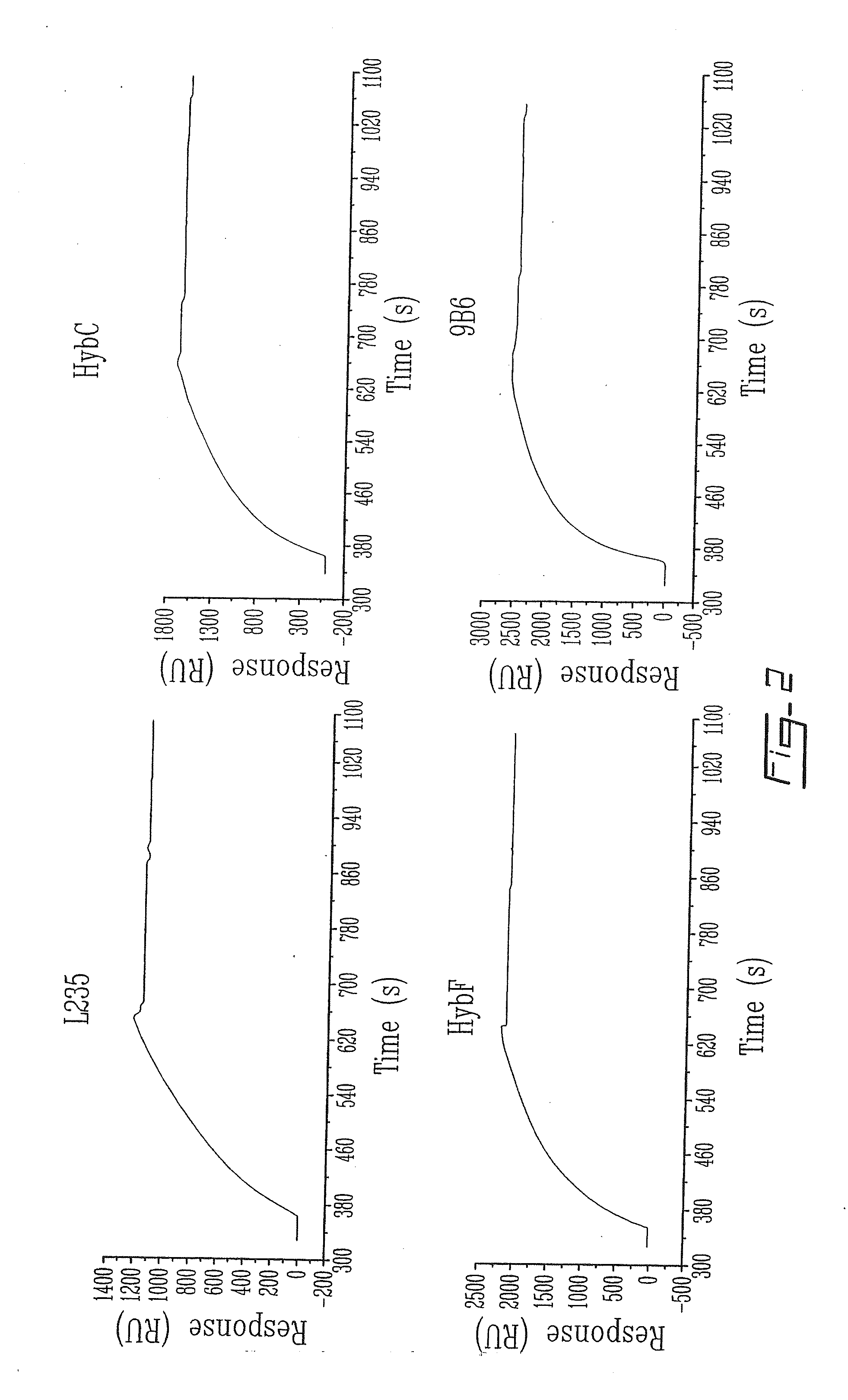
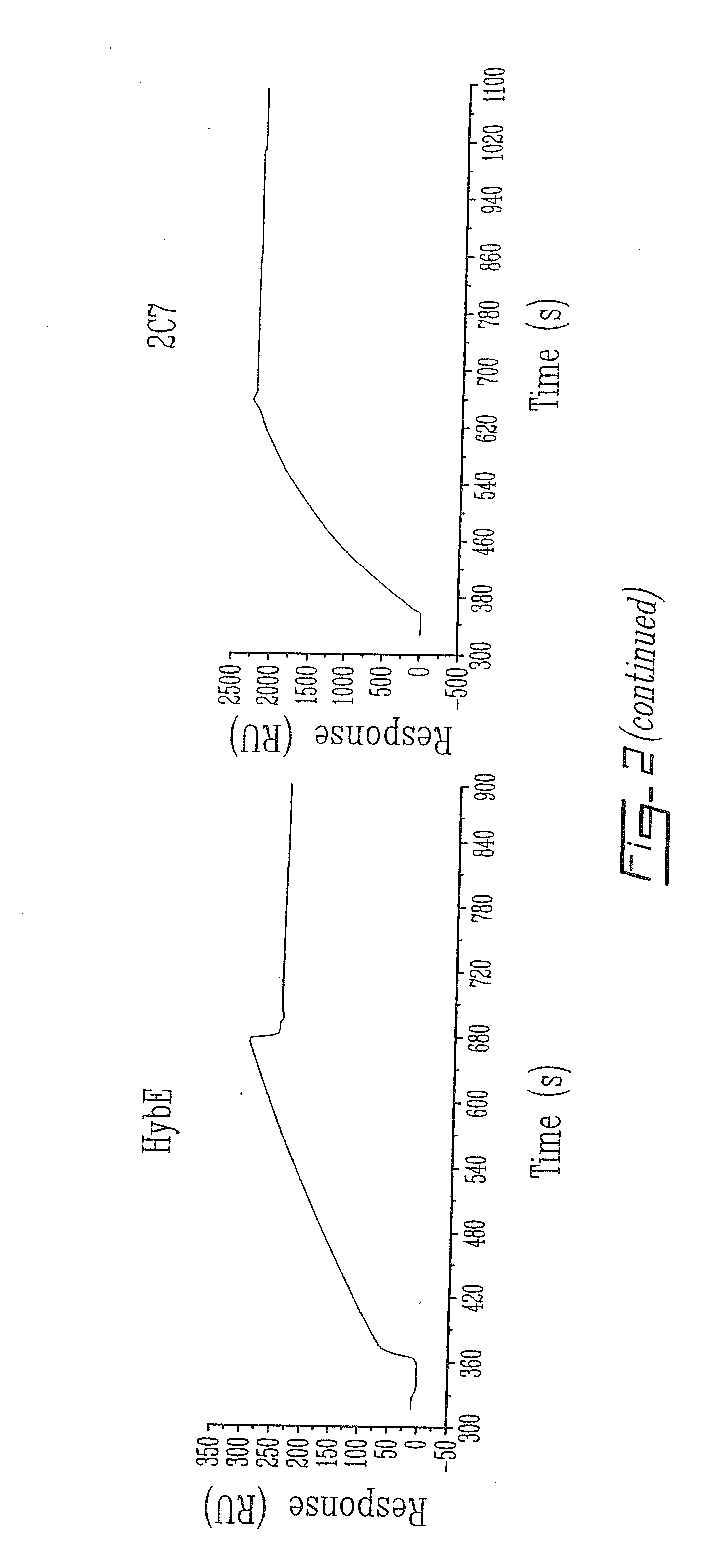
[0100]Biospecific interaction analysis in real-time between p97 and various anti-p97 mAbs is performed as follows. p97 is immobilized on a sensor chip (CM5) using standard coupling procedures incorporating NHS, EDC and ethanolamine. Different mAbs directed against p97 (HybC, HybE, HybF, L235, 2C7, 9B6), diluted to 0.05 μg / μl in Ringer / Hepes, are injected into the BIAcore at a flow rate of 5 μl / min. The surface plasmon resonance response obtained for these mAbs is plotted (in relative units (RU)) as a function of time. After each injection immobilized p97 is regenerated with 0.2M glycine at pH 2 for 2 min (n=4).
[0101]To evaluate the impact of immobilization procedures on the structural integrity of p97 different mAbs directed against various conformational epitopes of p97 were injected over p97 (FIG. 2). The surface plasmon resonance (SPR) signal generated by the interaction between p97 and...
example iii
Plasminogen Activation by p97
[0108]The interaction of p97 with pro-uPA was further characterized by measuring the activation of plasminogen by pro-uPA in the presence of p97 (FIG. 5). The plasminolytic activity of 1 nM uPA was measured without (o) or with () 70 nM p97 in the presence of 30 nM plasminogen. The reaction was performed in a final volume of 200 μl as described in the Materials and Methods section above. As a control, the enzymatic activity in the presence of p97 alone was also measured (▪). When p97 is added to pro-uPA and plasminogen, the VLK-pNA hydrolysis is 4-fold higher after 180 min (FIG. 5A). Control experiments performed with p97 indicated that this protein alone does not generate plasmin when it is added to plasminogen.
[0109]The plasmin activity in the presence of various concentrations of p97 was also measured (FIG. 5B). Plasmin activity induced by pro-uPA was measured in the presence of various p97 concentrations. Since the generation of plasmin proceeds at a...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com