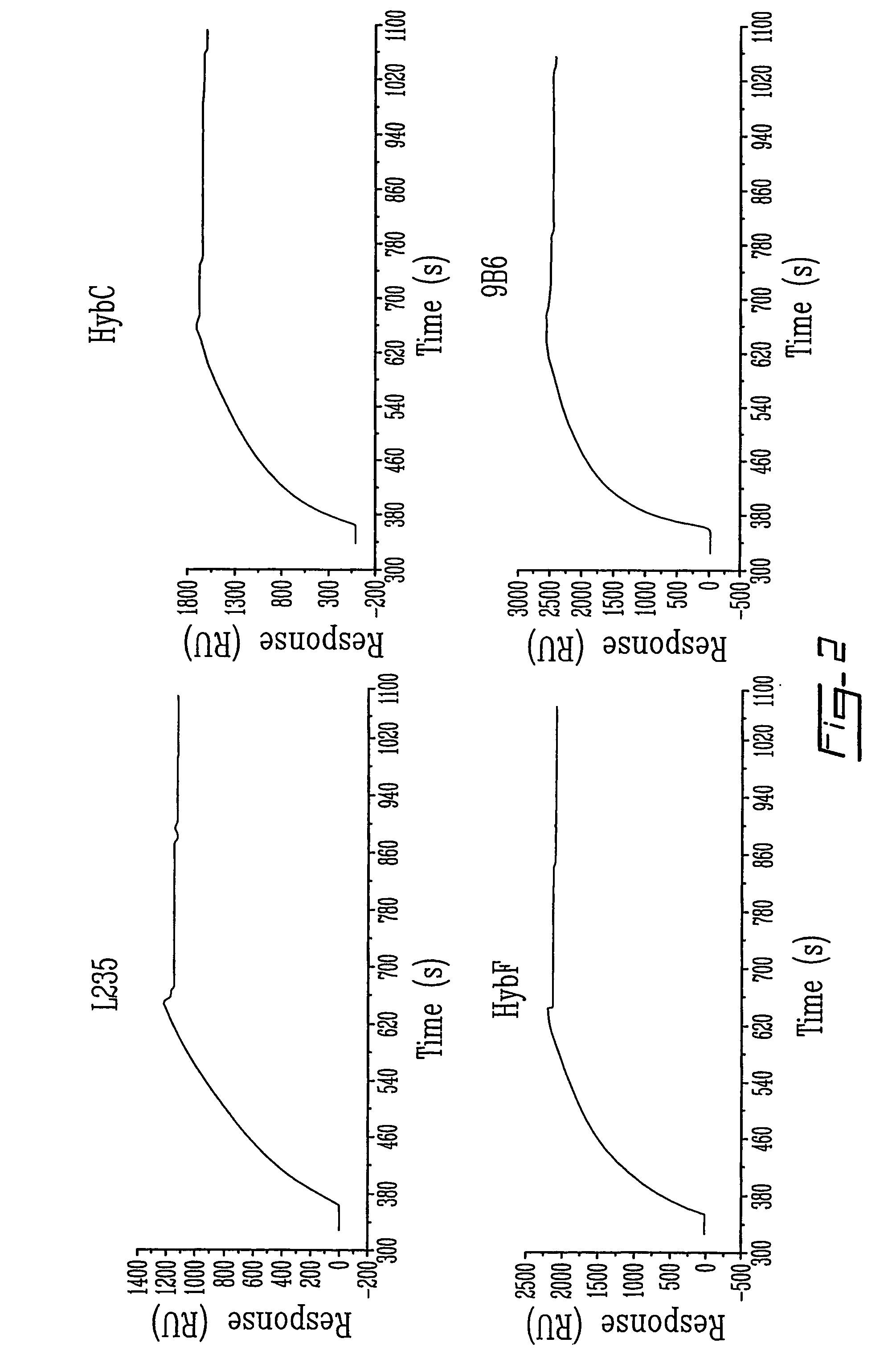
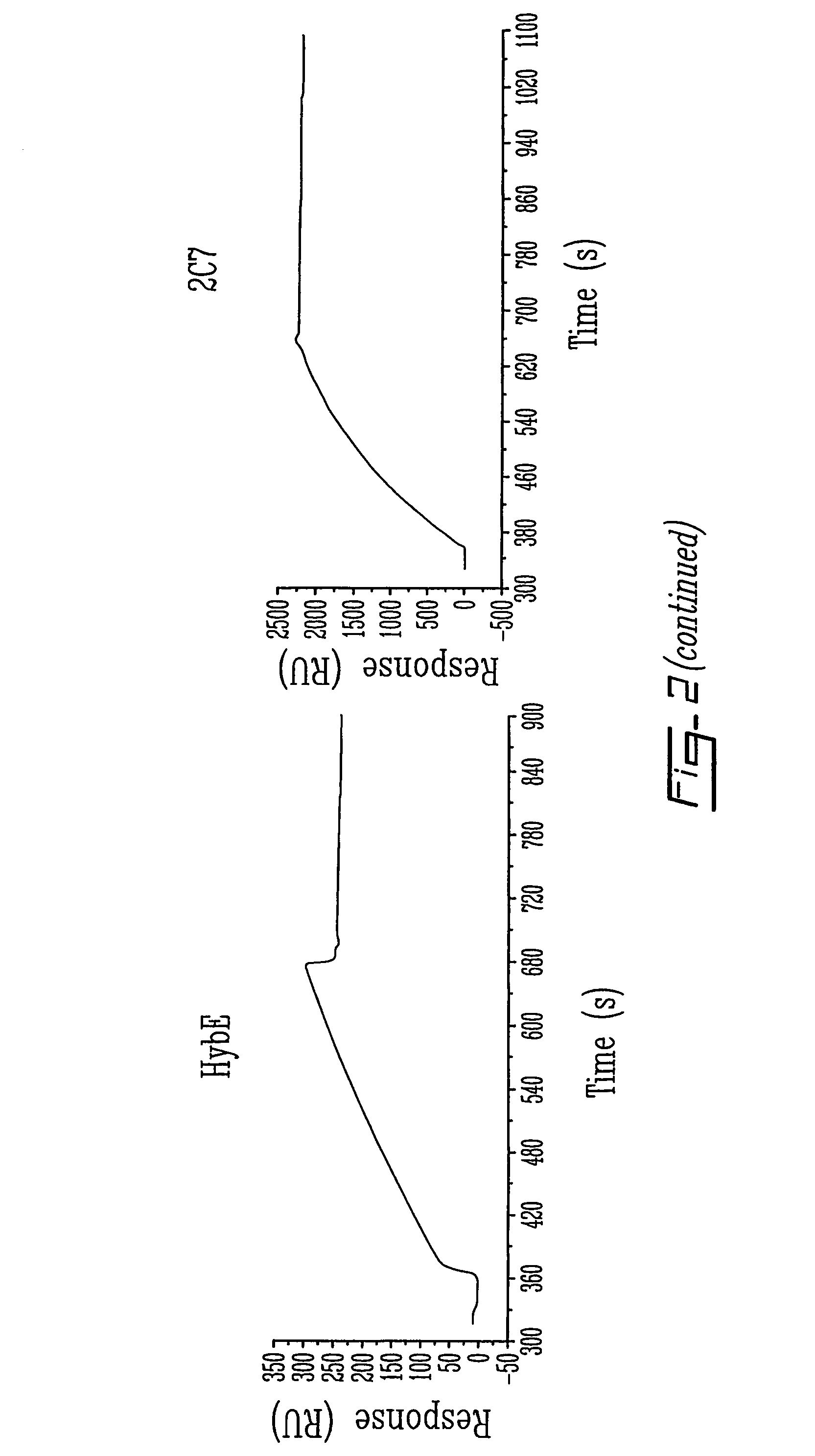
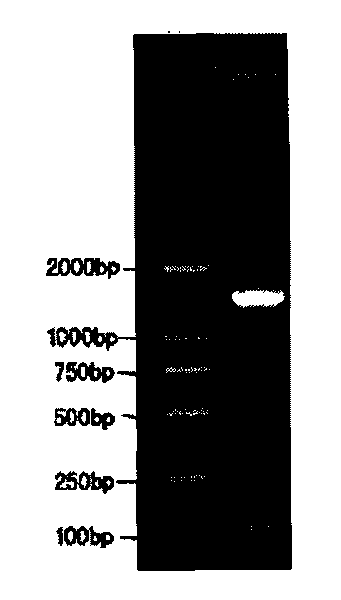
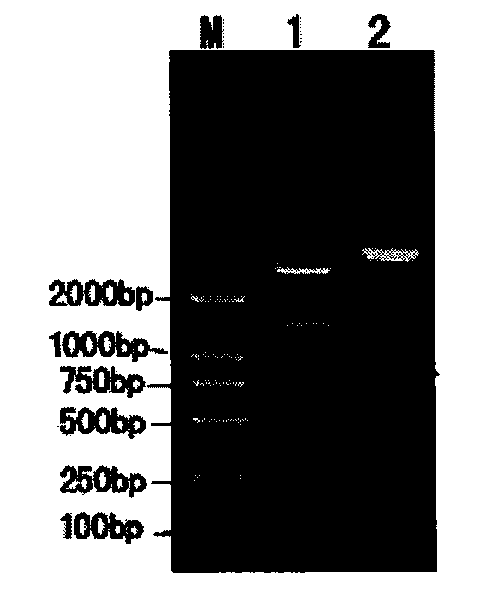
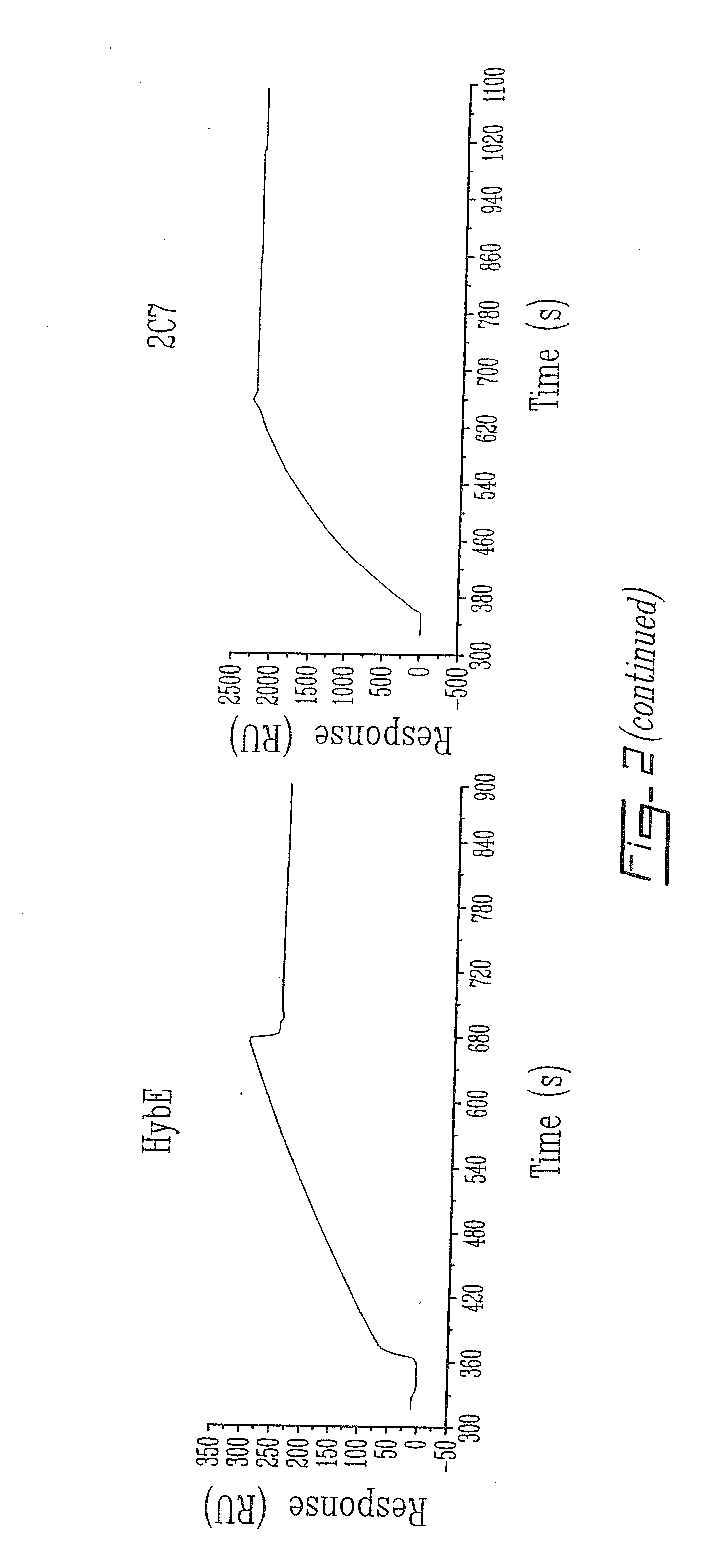
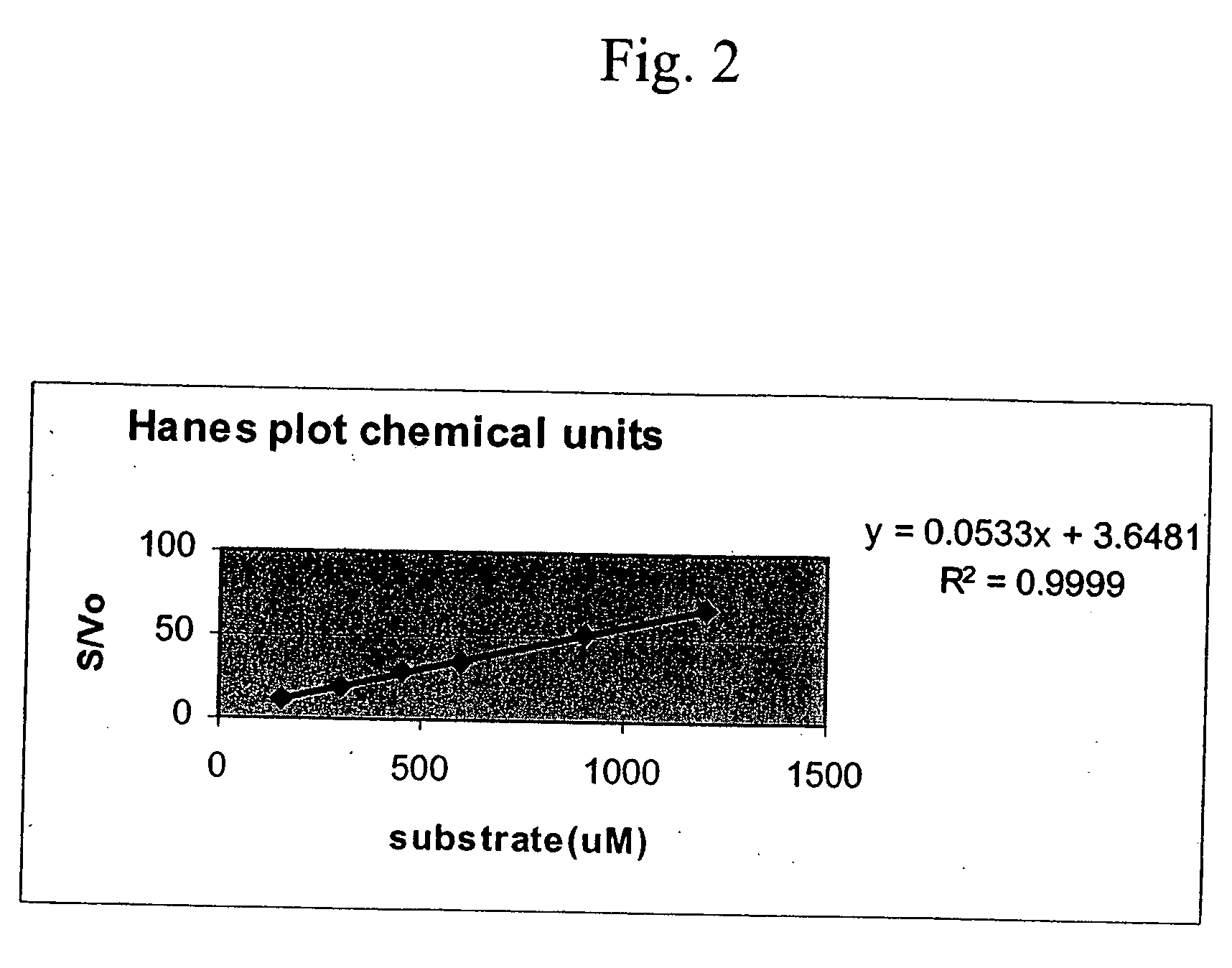
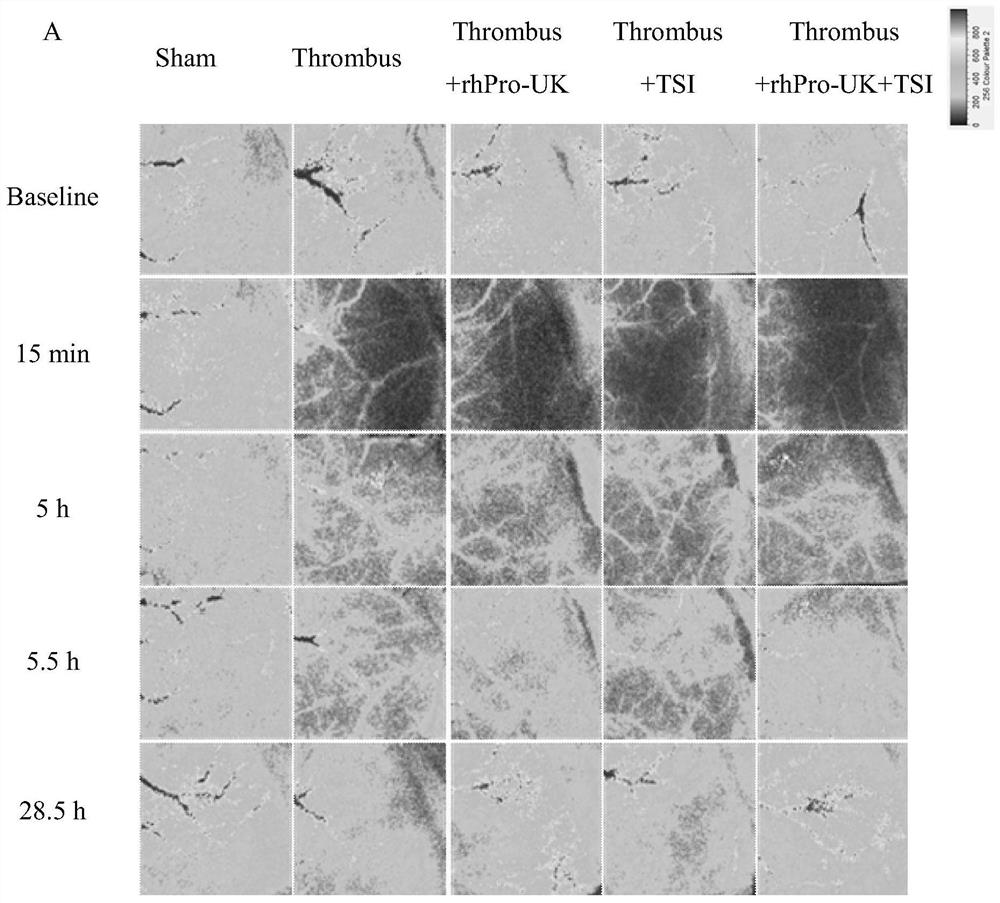
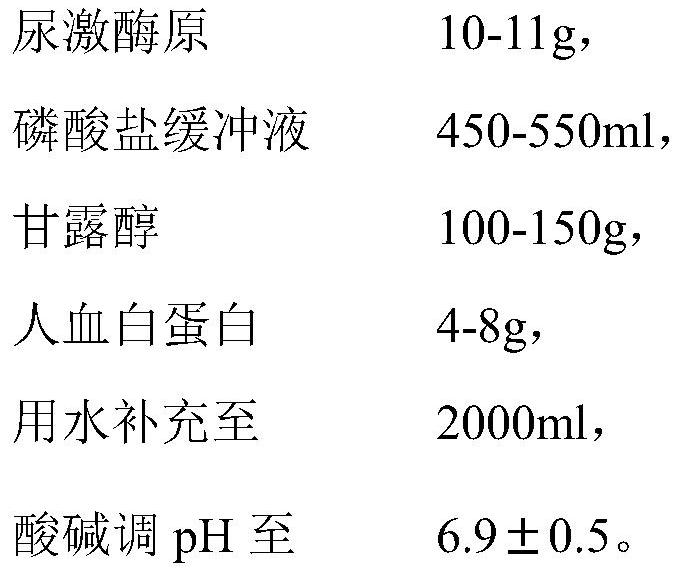
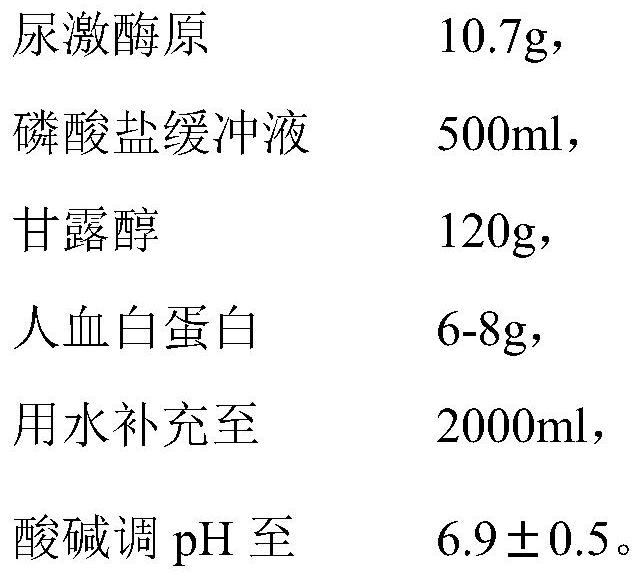
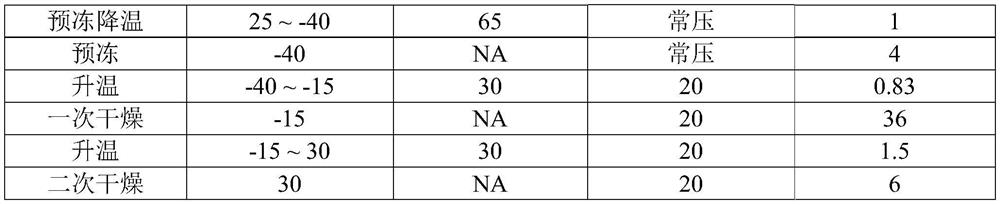
Patents
Literature
33 results about "Pro-urokinase" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
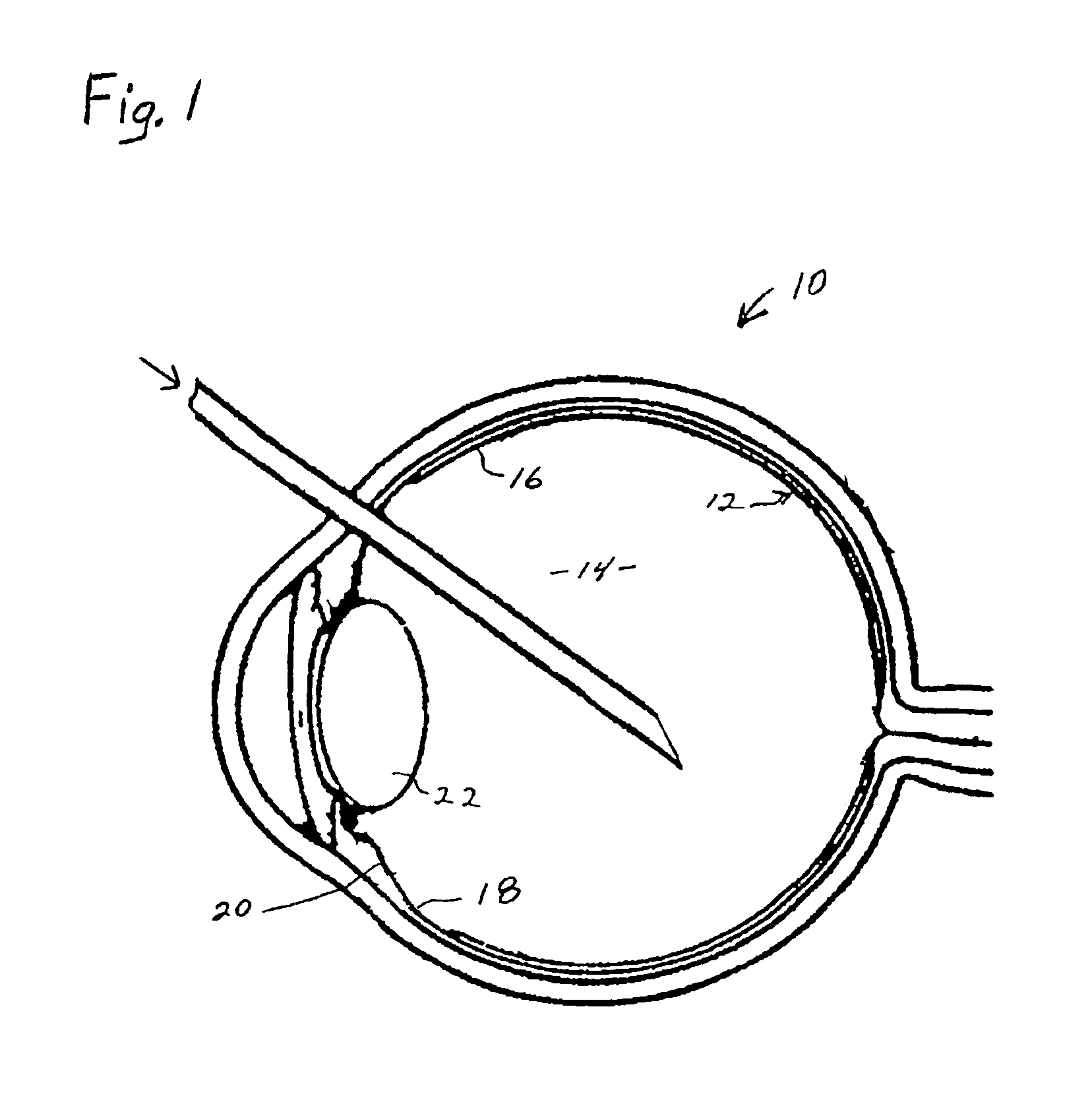
Process for generating plasmin in the vitreous of the eye and inducing separation of the posterior hyaloid from the retina
InactiveUS6899877B2Inhibiting retinal tearReducing and preventing effectSenses disorderEye implantsUrokinase Plasminogen ActivatorVascular proliferation
A process for inhibiting vascular proliferation separately introduces components into the eye to generate plasmin in the eye in amounts to induce complete posterior vitreous detachment where the vitreoretinal interface is devoid of cortical vitreous remnants. The process administers a combination of lysine-plasminogen, at least one recombinant plasminogen activator selected from the group consisting of urokinase, streptokinase, tissue plasminogen activator, chondroitinase, pro-urokinase, retavase, metaloproteinase, and thermolysin and a gaseous adjuvant to form a cavity in the vitreous. The composition is introduced into the vitreous in an amount effective to induce crosslinking of the vitreous and to induce substantially complete posterior vitreous detachment from the retina without causing inflammation of the retina. The gaseous adjuvant material is introduced into the vitreous simultaneously with or after the lysine-plasminogen and recombinant plasminogen activator such as recombinant urokinase to compress the vitreous against the retina while the composition induces the complete posterior vitreous detachment.
Owner:MINU
Purification of recommbined human urokinase zymogen
ActiveCN1680550AEasy to fillLarge amount of processingPeptide preparation methodsPeptidasesZymogenUrokinase Plasminogen Activator
The invention is about a method to purify the recombined human urokinase by the chromatography. The invention relates to recovery the gene engineering production from the mammal cell culture using the Streamline-SP, Sephacryl S-200, Sepharose Fast Flow and DEAE-Sepharose Fast Flow method. The recombined human urokinase can meet the SFDA standard and the percent recovery is above 70%, the purity is above 99% by using the method.
Owner:TASLY BIOPHARMACEUTICALS CO LTD
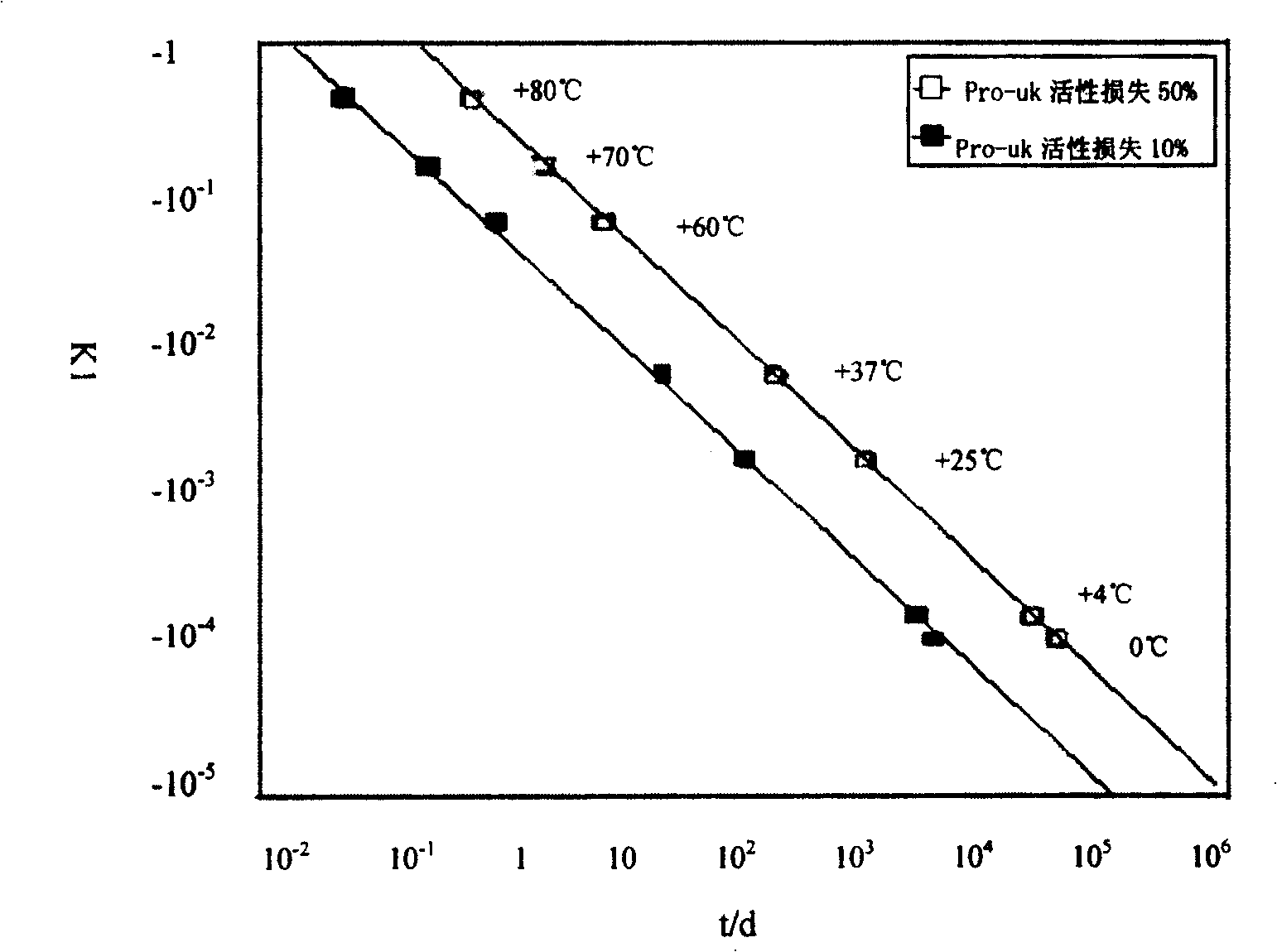
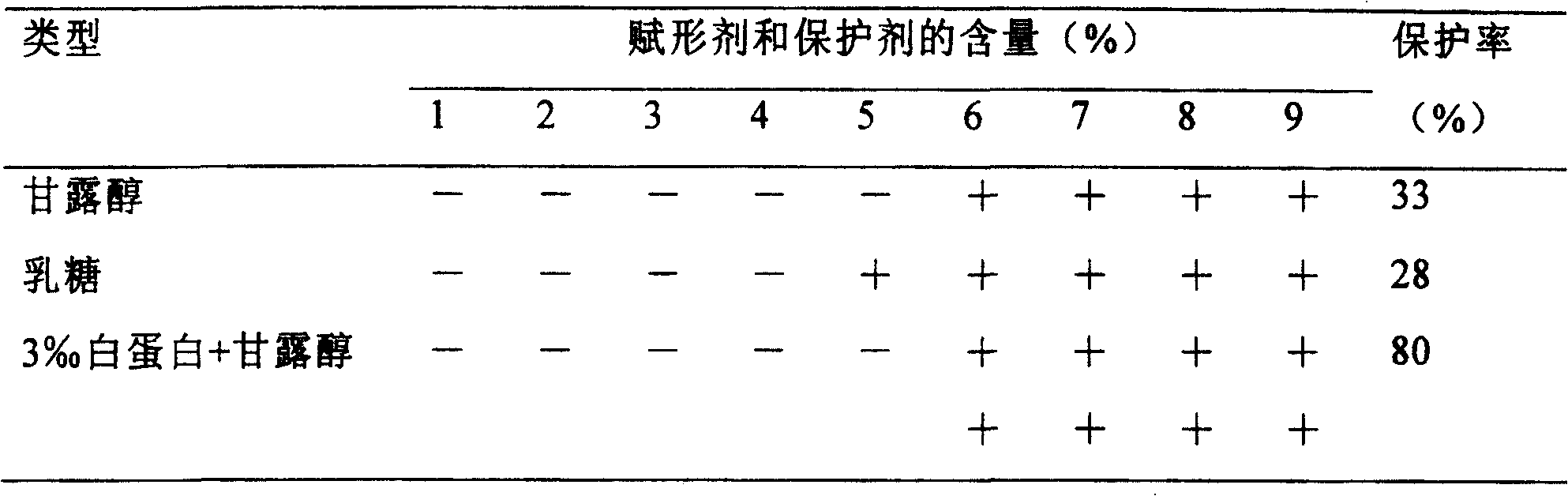
Composition containing active prourokinase, freeze-drying process and freeze-dried preparation thereof
ActiveCN1730098AFor long-term storageLow pricePowder deliveryPeptide/protein ingredientsFreeze-dryingPro-urokinase
Owner:TASLY BIOPHARMACEUTICALS CO LTD
Method for manufacturing adjustable liver injury animal model and special carriers thereof
ActiveCN101550425AImprove survival rateReduce difficultyVector-based foreign material introductionAnimal husbandryPro-urokinaseCloning Site
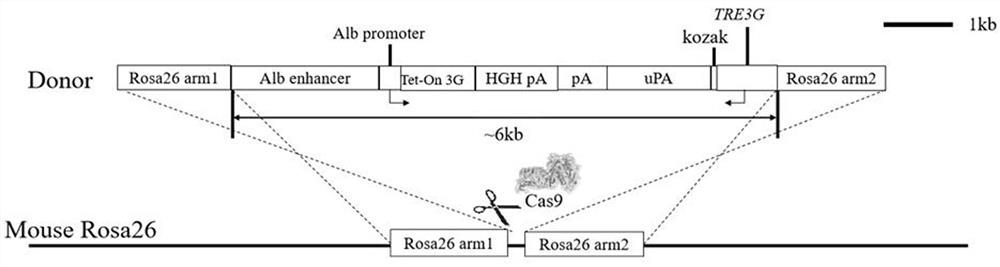
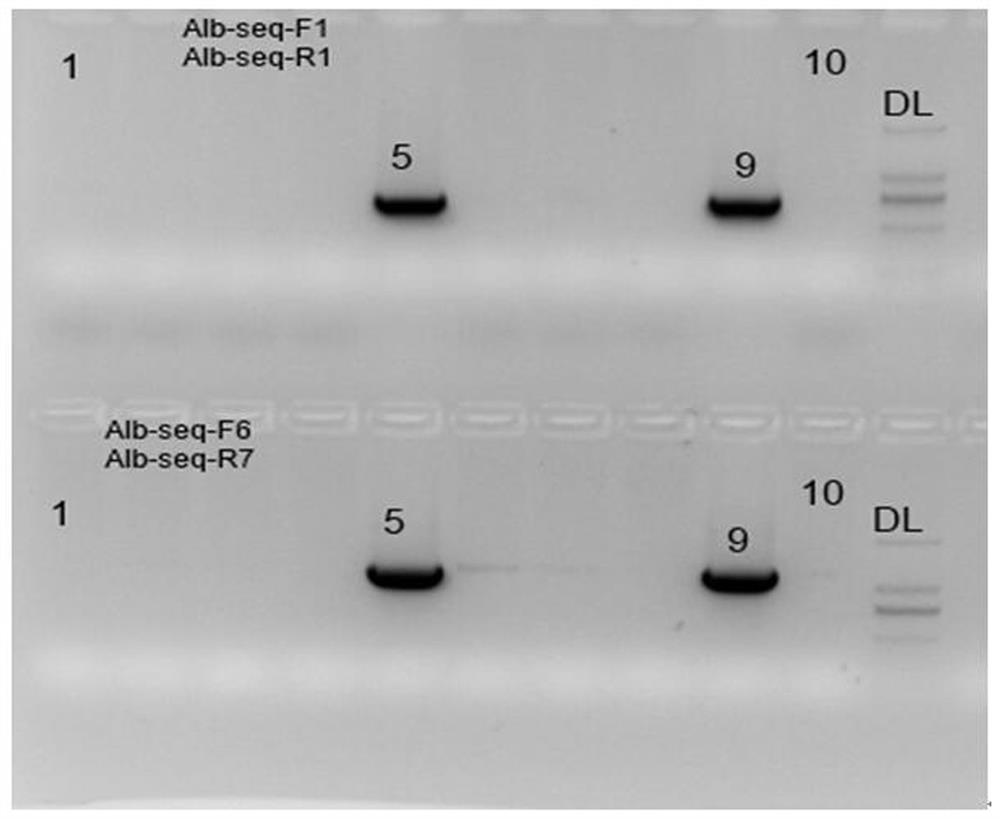
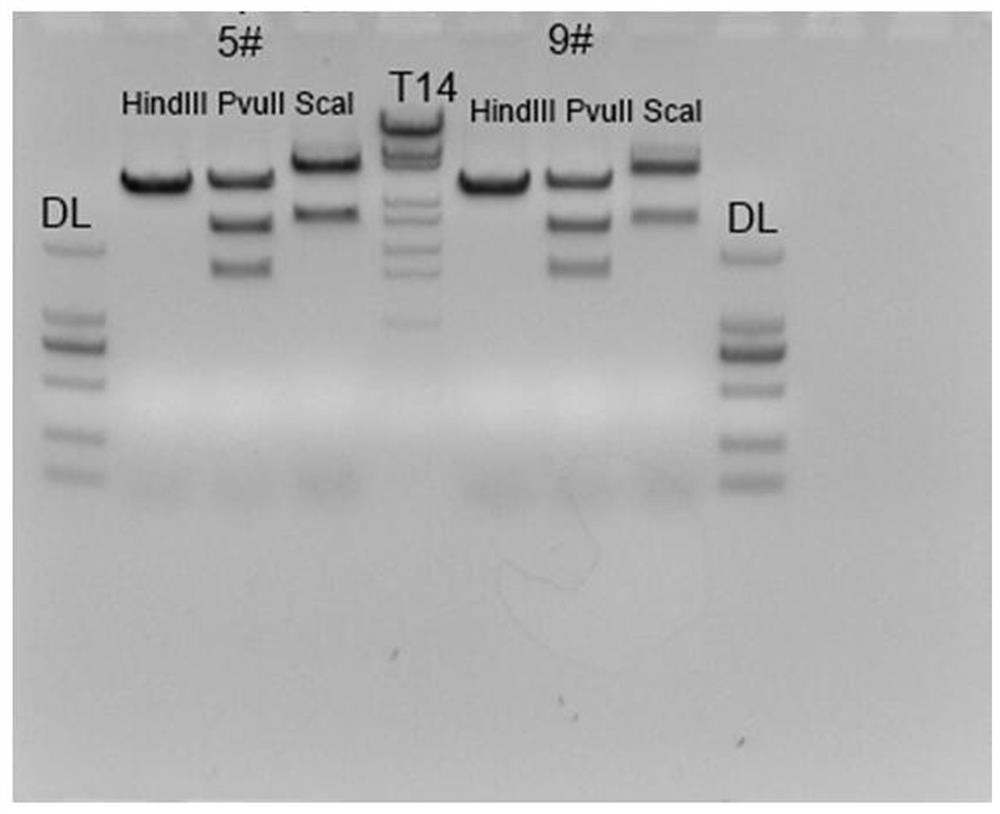
The invention discloses a method for manufacturing an adjustable liver injury animal model and special carriers thereof. The invention provides two special carriers; one is obtained by inserting DNA fragments formed by sequentially connecting tetracycline reacting factors, promoters and pro-urokinase activator coding genes of a Tet-off / Tet-on gene expression system from the upper stream to the lower stream into the multiple cloning sites of an eukaryotic expression carrier; and the other one is obtained by inserting DNA fragments formed by sequentially connecting liver special promoters and antisense tetracycline activator coding genes of a Tet-on gene expression system from the upper stream to the lower stream into the multiple cloning sites of an eukaryotic expression carrier. The two special carriers re implanted into the animal bodies for obtaining a double-positive transgenic animal model, and the expression time and the expression quantity of uPA genes in the liver cells of the animal model are adjusted through the induction of doxycline, thereby the time and the degree of the liver injury can be manually controlled according to demands.
Owner:PEKING UNIV +1
Vector of uPA expression regulated by Tet-on system and application thereof
InactiveCN101948860AEasy constructionVector-based foreign material introductionAnimal husbandryUrokinase Plasminogen ActivatorNucleotide
The invention discloses an effect vector of uPA expression regulated by a Tet-on system. The nucleotide sequence of the effect vector comprises a bacteria tetracycline drug-resistant operon sequence, an immediate early promoter sequence of macrophages, a mice pro-urokinase activator expression cassette sequence and a sequence behind the last exon of the mice pro-urokinase activator expression cassette, the four sequences are serially connected in turn. The invention establishes a transgenic mice model of uPA expression regulated by the Tet-on system or tetracycline derivative inducible expression system, and lays solid foundation for further establishing a transgenic animal model of tissue specificity regulatable expression uPA for uPA function research in specific tissues.
Owner:MICROBE EPIDEMIC DISEASE INST OF PLA MILITARY MEDICAL ACAD OF SCI
Targeting vector and nucleic acid composition for constructing liver injury mouse model, and construction method
ActiveCN111733183AReliable screeningReaction metabolismVectorsStable introduction of DNADiseasePro-urokinase
The invention discloses a targeting vector and nucleic acid composition for constructing a liver injury mouse model, and a construction method, and relates to the technical field of genetic engineering and genetic modification. The targeting vector disclosed by the invention comprises a first expression cassette and a second expression cassette positioned at the downstream of the first expressioncassette; the first expression cassette comprises the following sequential series components: a liver-specific promoter, a tetracycline transcriptional activation regulating factor and a first polyA;and the second expression cassette comprises the following sequential series components: a second polyA, a mouse prourokinase activator coding gene, and a tetracycline-induced promoter. The liver injury mouse model constructed by the targeting vector has henotype of spontaneous liver injury and induction aggravated liver injury; the mouse death rate of the filial generation thereof is low; large-scale breeding can be conveniently carried out; and the invention provides the reliable liver injury mouse model for researching liver diseases.
Owner:GEMPHARMATECH CO LTD
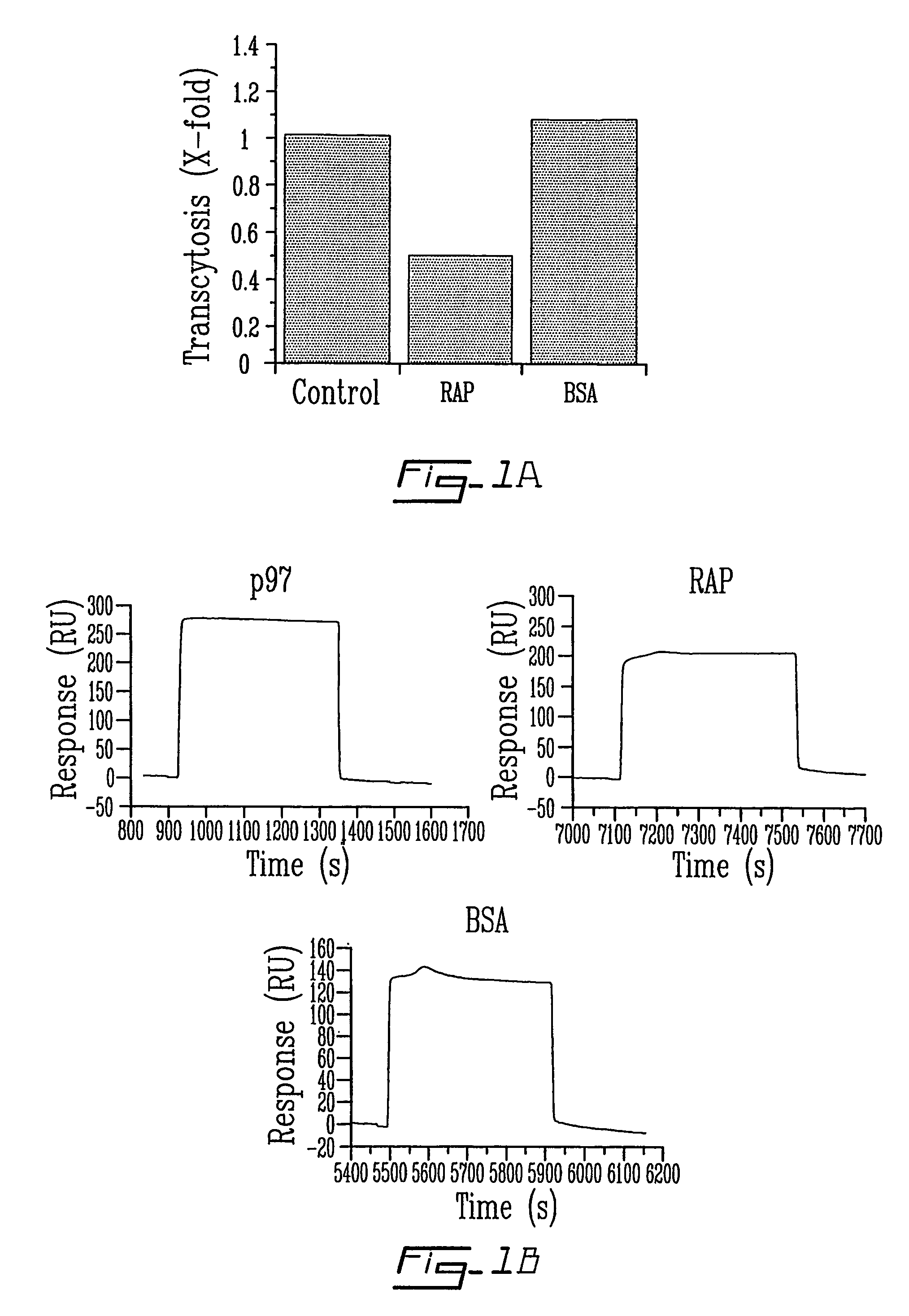
Compound and method for regulating plasminogen activation and cell migration
InactiveUS7919103B2Prevents and reduces capillary tube formationPeptide/protein ingredientsSnake antigen ingredientsDiseaseAngiogenesis growth factor
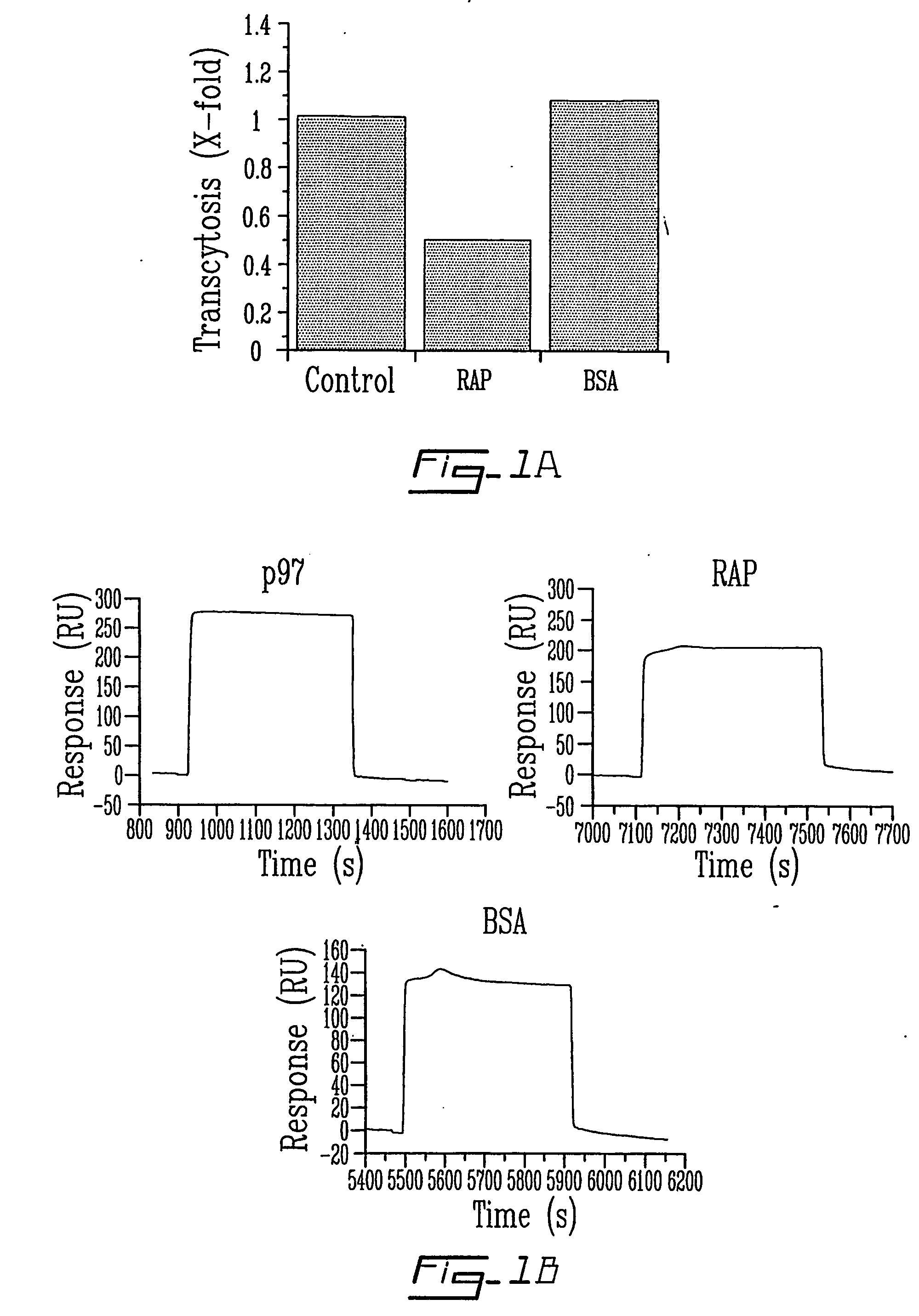
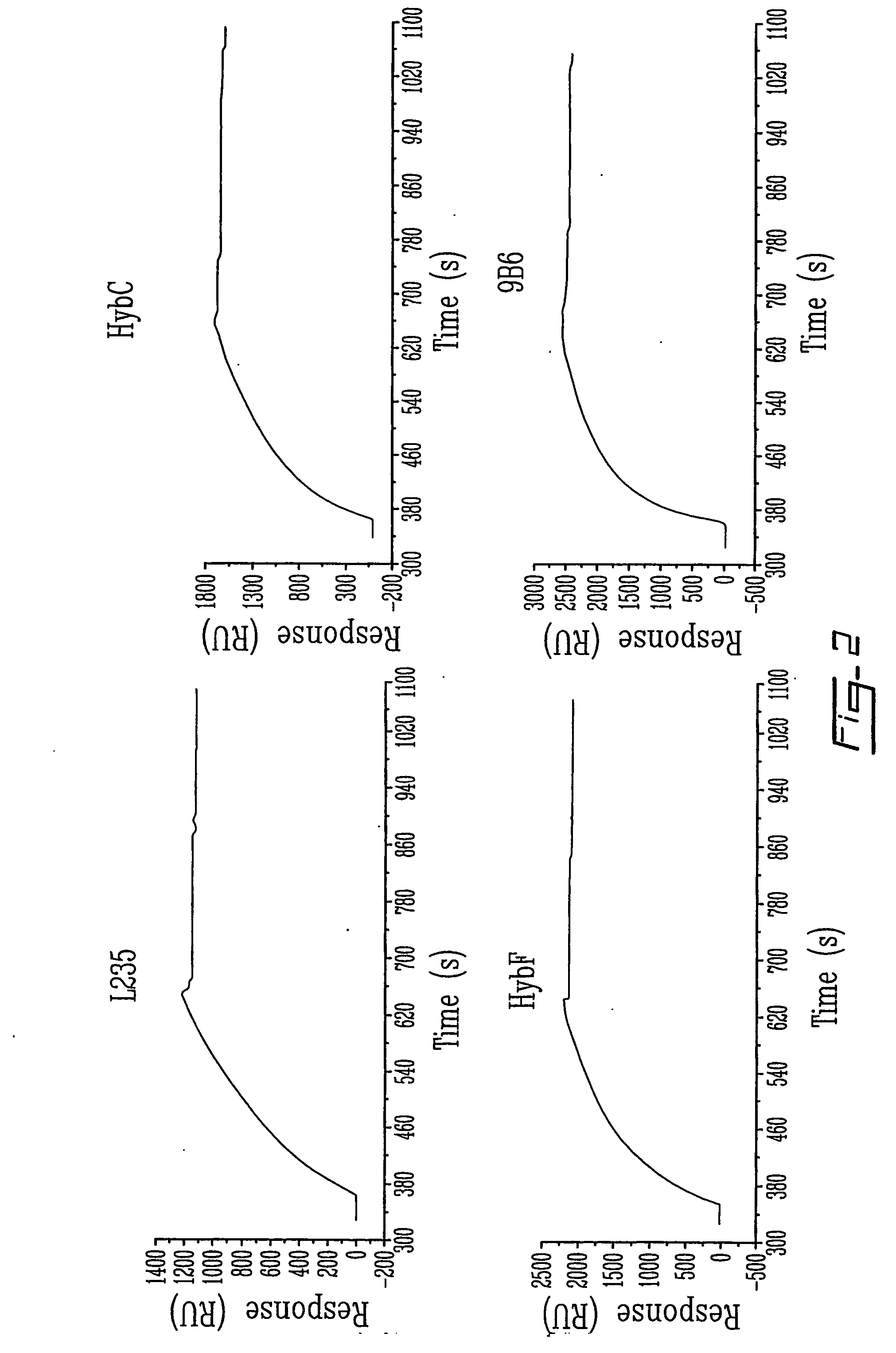
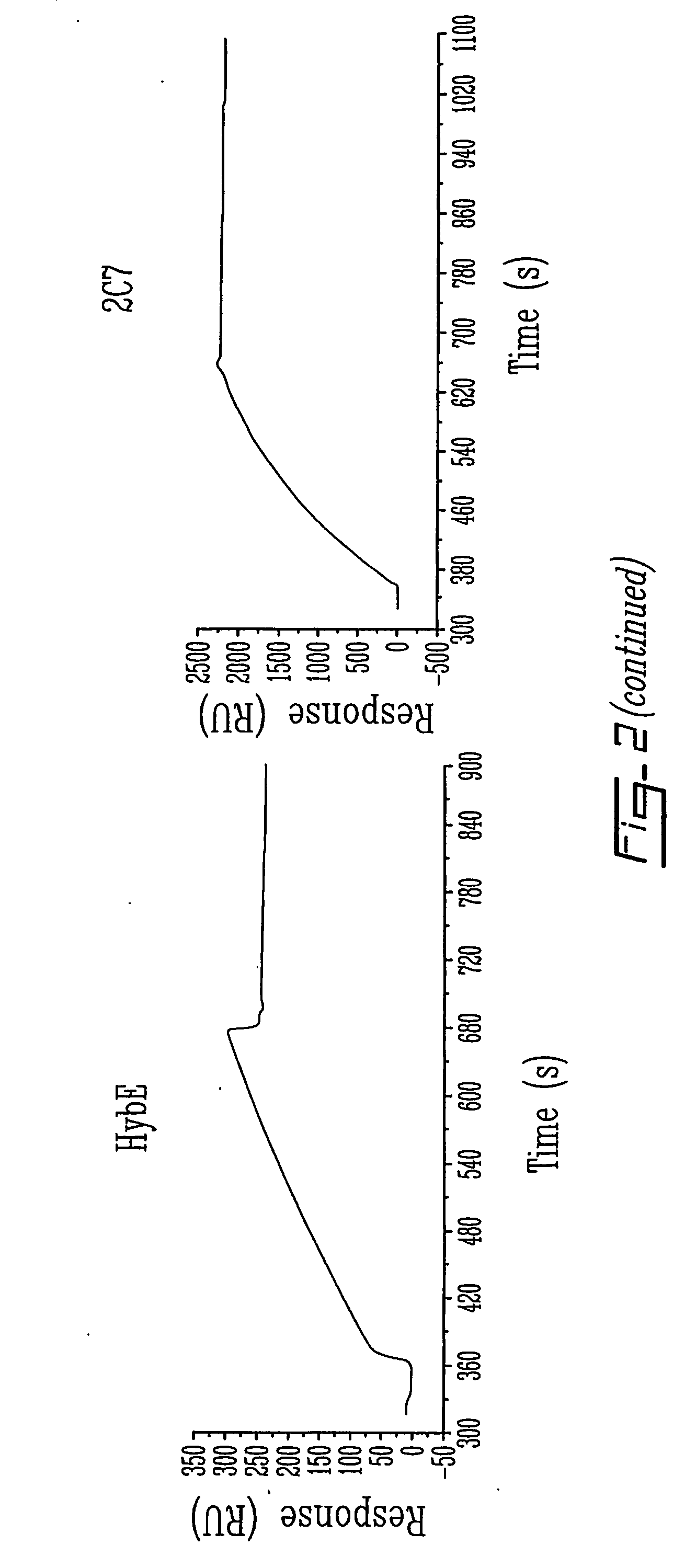
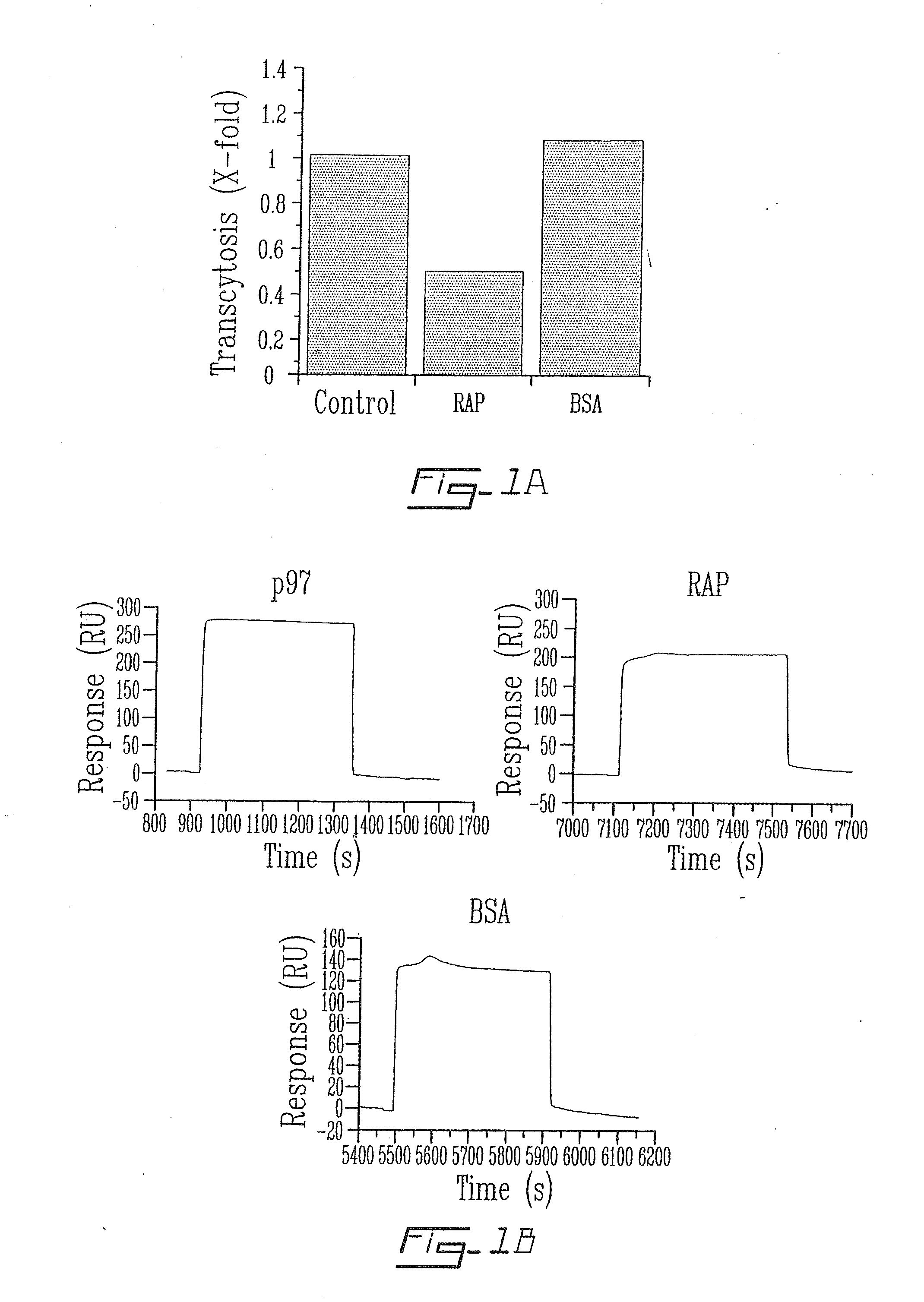
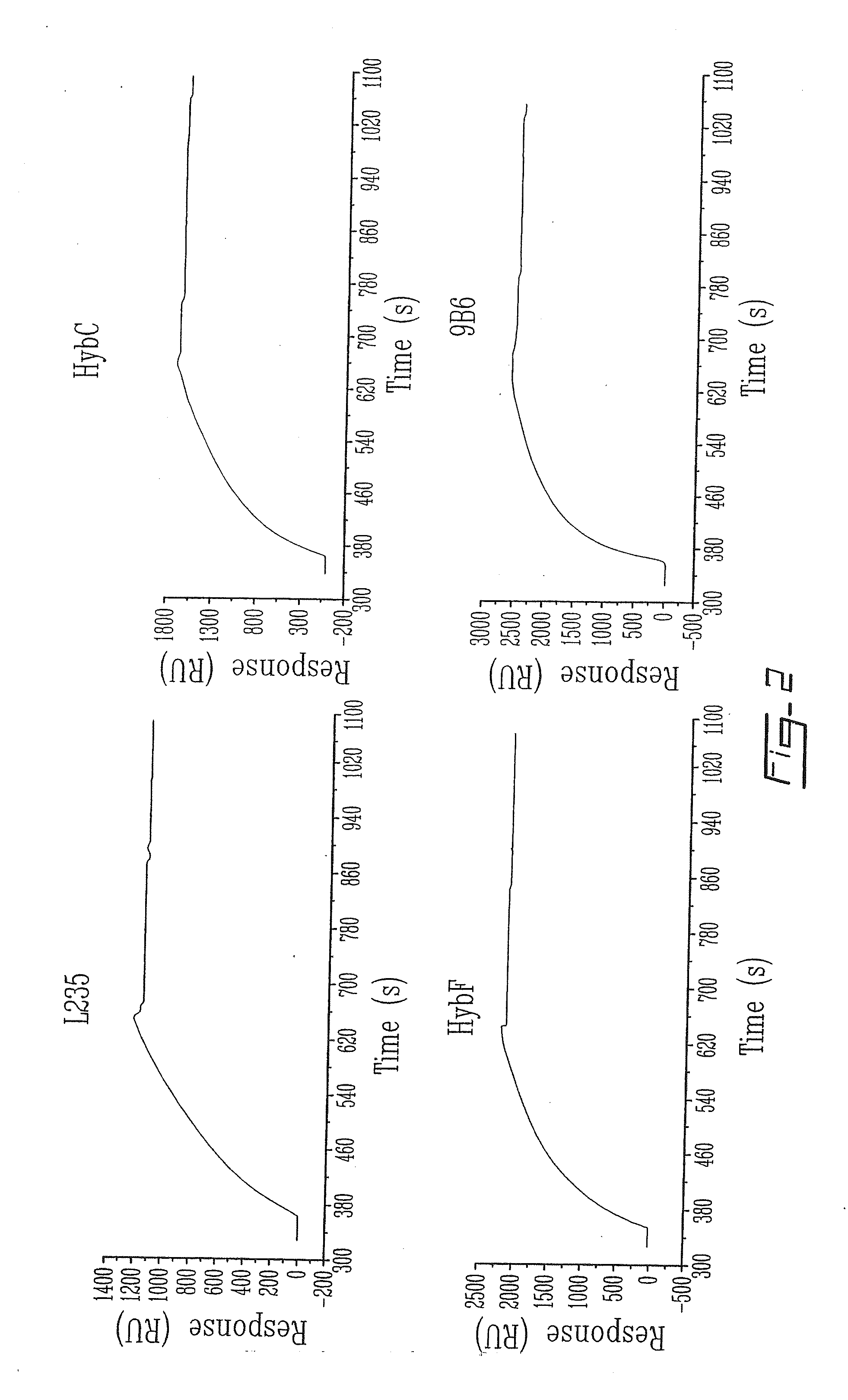
The invention relates to novel regulators of plasminogen activation and their use for regulating cell migration, plasminolysis, angiogenesis, fibrinolysis, for treating cancer and thrombo-embolic diseases such as heart stroke. Furthermore, the present invention relates to novel pharmaceutical compositions form regulating cell migration, plasminolysis, angiogenesis and for treating cancer. In particular, the present invention relates to a method of regulating the activation of plasminogen comprising contacting a solution of pro-urokinase (uPA) or tissue plasminogen activator (tPA) and plasminogen with melanotransferrin (p97) for a time sufficient to effect regulation thereof.
Owner:TRANSFERT PLUS
Manufacturing method of adjustable liver damage animal model and special DNA fragment thereof
The invention discloses a manufacturing method of an adjustable liver damage animal model and a special DNA fragment thereof; a DNA fragment I is formed by sequentially connecting liver super promoters and antisense tetracycline activator coding genes of a Tet-on gene expression system from upstream to downstream; an DNI fragment II is formed by sequentially connecting tetracycline reaction factors, promoters and prourokinase activator coding genes of the Tet-off / Tet-on gene expression system from upstream to downstream; a recombination expression vector carrying the DNI fragment II is used for converting mammalian cells, the cells are cultivated to obtain recombinant adenovirus. The DNA fragment I is led in the animal to obtain a transgenosis first-construction animal; the transgenosis first-construction animal and scid / bg animal are back-crossed to obtain the animal carrying the DNA fragment I with the scid / bg background; the recombinant adenovirus is used for injecting the animal carrying the DNA fragment I with the scid / bg background, so as to obtain the adjustable liver damage animal model; in the model, the prourokinase activator expression is activated by doxycycline, the expression intensity is changed along with the change of dosage, the specificity is very high.
Owner:PEKING UNIV
Method for producing recombinaton urokinase
InactiveCN1537939APeptide/protein ingredientsPeptide preparation methodsRecombinant UrokinaseUrokinase Plasminogen Activator
Highly efficient methods of producing properly folded recombinant urokinase are provided. Denatured recombinant pro-urokinase is refolded by first solubilizing the protein with a chaotroph at high pH, followed by refolding in the presence of reduced concentrations of chaotroph while the pH is slowly reduced.
Owner:PROTEOMTECH
Compound and method for regulating plasminogen activation and cell migration
InactiveUS20070053894A1Prevents and reduces capillary tube formationDecrease and prevents and delayPeptide/protein ingredientsSnake antigen ingredientsDiseaseBiological activation
The invention relates to novel regulators of plasminogen activation and their use for regulating cell migration, plasminolysis, angiogenesis, fibrinolysis, for treating cancer and thrombo-embolic diseases such as heart stroke. Furthermore, the present invention relates to novel pharmaceutical compositions form regulating cell migration, plasminolysis, angiogenesis and for treating cancer. In particular, the present invention relates to a method of regulating the activation of plasminogen comprising contacting a solution of pro-urokinase (uPA) or tissue plasminogen activator (tPA) and plasminogen with melanotransferrin (p97) for a time sufficient to effect regulation thereof.
Owner:TRANSFERT PLUS
Compound and method for regulating plasminogen activation and cell migration
InactiveUS20110243952A1Prevents and reduces capillary tube formationPeptide/protein ingredientsImmunoglobulins against cell receptors/antigens/surface-determinantsDiseaseAngiogenesis growth factor
The invention relates to novel regulators of plasminogen activation and their use for regulating cell migration, plasminolysis, angiogenesis, fibrinolysis, for treating cancer and thrombo-embolic diseases such as heart stroke. Furthermore, the present invention relates to novel pharmaceutical compositions form regulating cell migration, plasminolysis, angiogenesis and for treating cancer. In particular, the present invention relates to a method of regulating the activation of plasminogen comprising contacting a solution of pro-urokinase (uPA) or tissue plasminogen activator (tPA) and plasminogen with melanotransferrin (p97) for a time sufficient to effect regulation thereof.
Owner:TRANSFERT PLUS
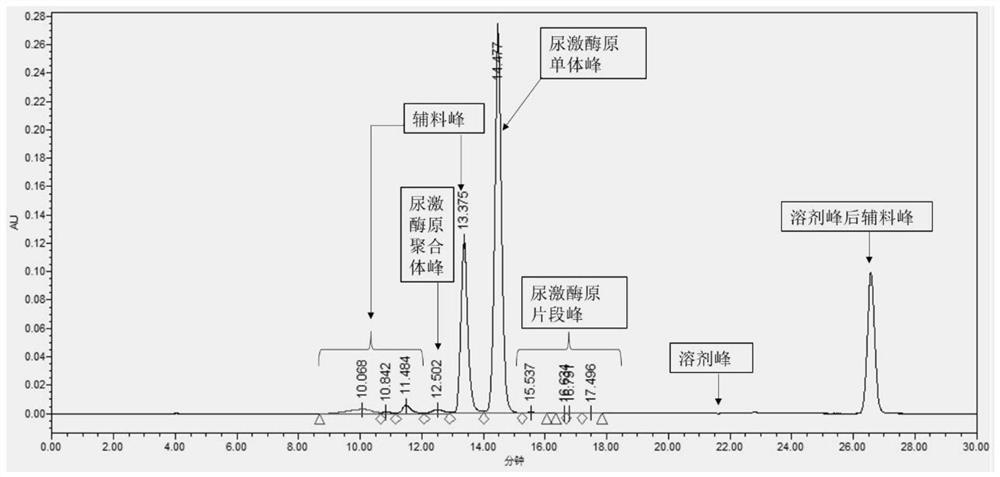
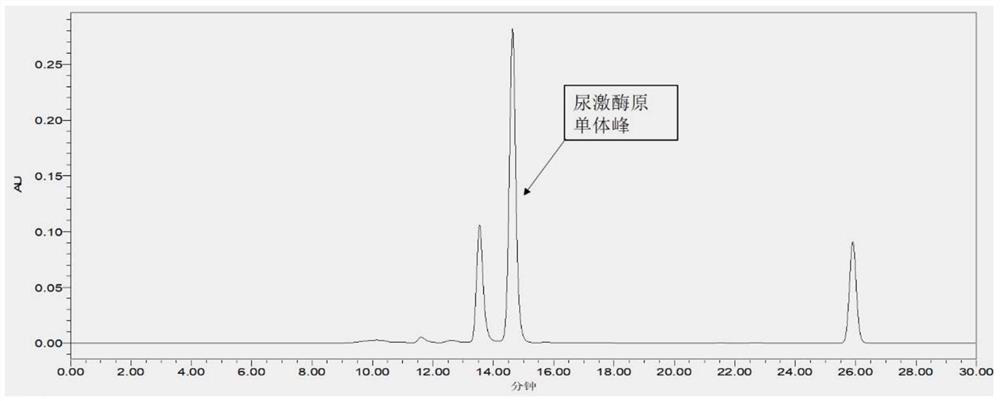
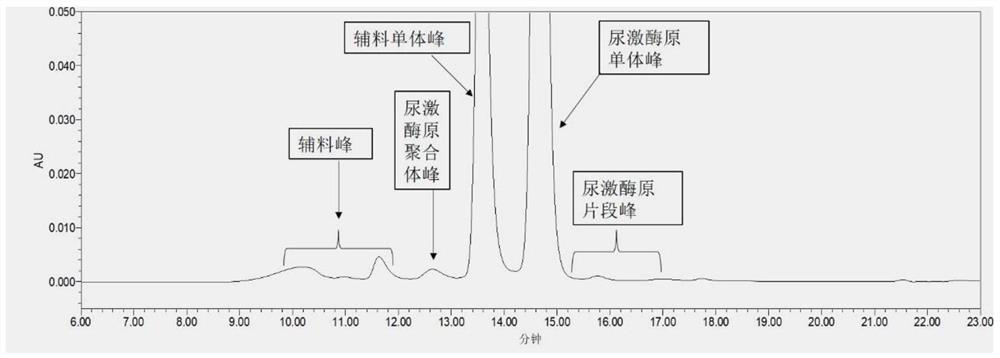
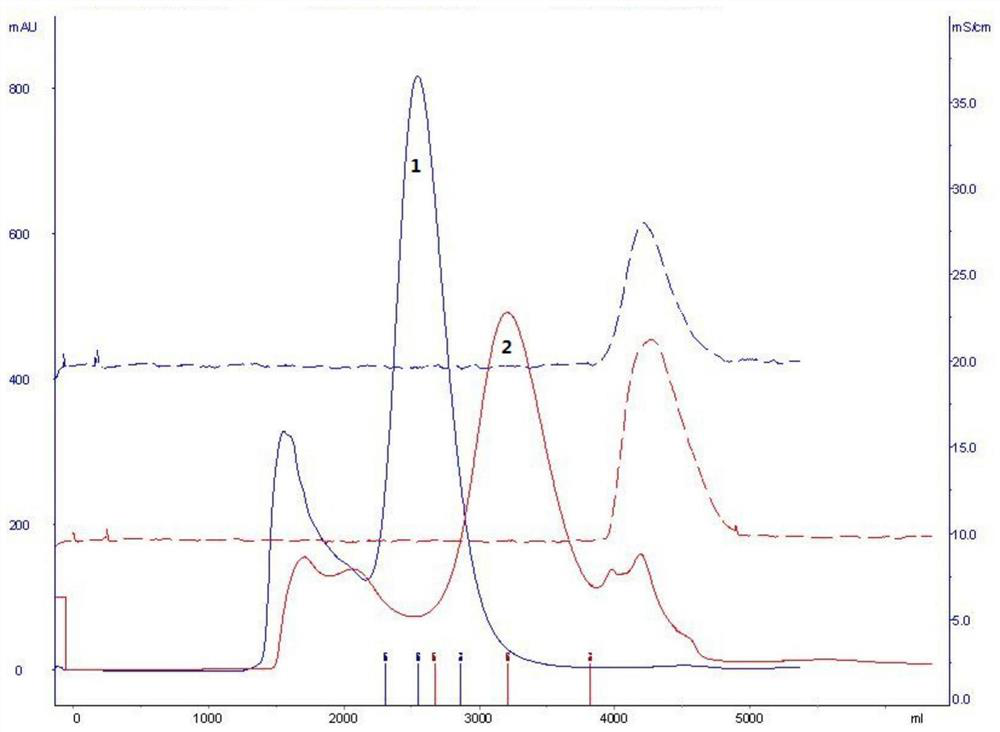
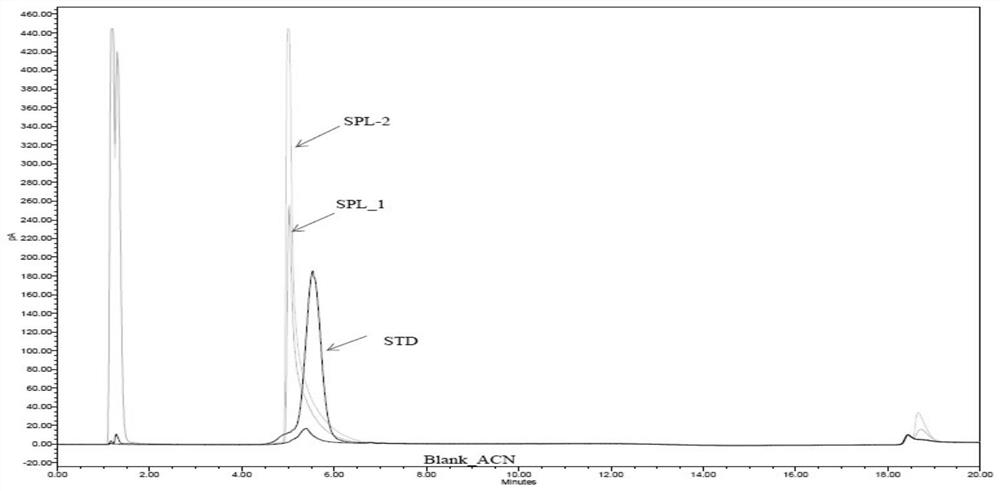
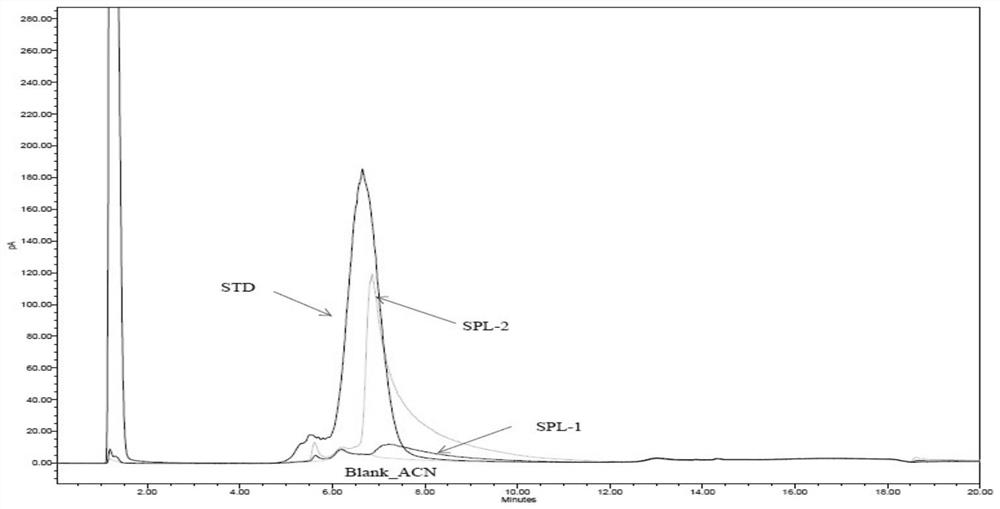
Method for detecting purity of recombinant human prourokinase
ActiveCN113671081AEasy to separateMeet the requirements of separationComponent separationAgainst vector-borne diseasesBiochemistryPro-urokinase
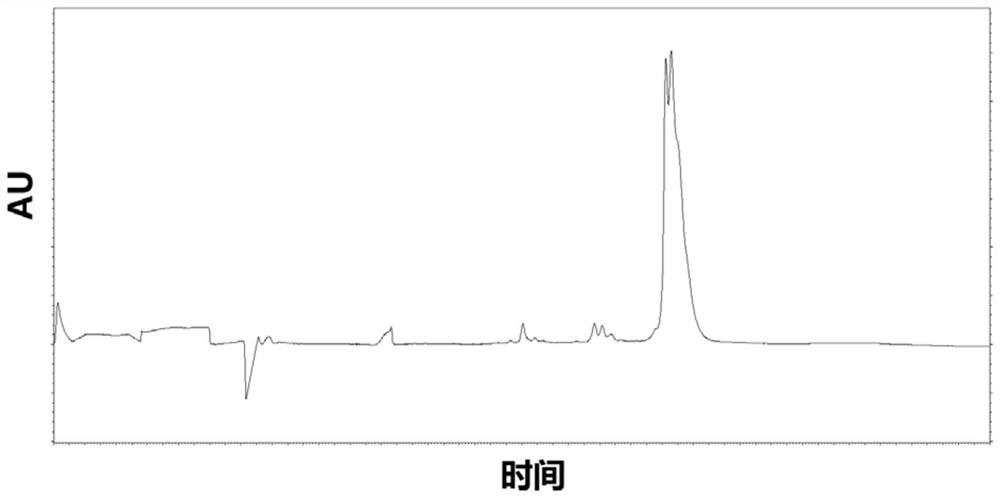
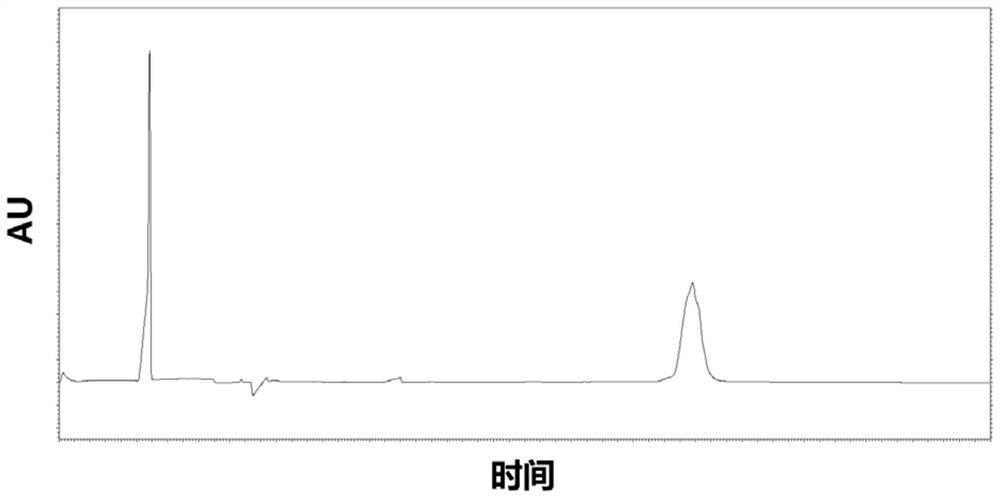
The invention provides a method for detecting the purity of recombinant human prourokinase. The method for detecting the purity of the recombinant human prourokinase comprises the following steps: diluting a recombinant human pro-urokinase sample into a sample solution, and detecting by adopting SEC-HPLC to obtain the purity of the sample, wherein a mobile phase A adopted by the SEC-HPLC is an aqueous solution of acid with the pH value of 2.5-3.5, and a mobile phase B adopted by the SEC-HPLC is acetonitrile. The detection method can be used for detecting the purity of a prourokinase finished product, is also suitable for analyzing the purity of a prourokinase stock solution, can accurately detect the prourokinase polymer in the finished product, and further improves the separation degree of the prourokinase polymer and the prourokinase monomer.
Owner:TASLY BIOPHARMACEUTICALS CO LTD
New use of gene reconstituted urokin as origin
InactiveCN1651077AReduce trafficStable flowPowder deliveryPeptide/protein ingredientsDiseaseUrokinase Plasminogen Activator
An application of gene-recombinant prourokinase in preparing medicines for treating acute myocardial infarction, cerebral infarction, pulmonary embolism, phlebothrombosis of leg, etc is disclosed.
Owner:TASLY BIOPHARMACEUTICALS CO LTD
Prourokinase modifier, preparation method, pharmaceutical composition, use, encoding gene, carrier containing gene and transformation cell
InactiveCN1924013ASpecific thrombolysisIncrease fibrinolytic activityPeptide/protein ingredientsEnzymesPeptideDrug
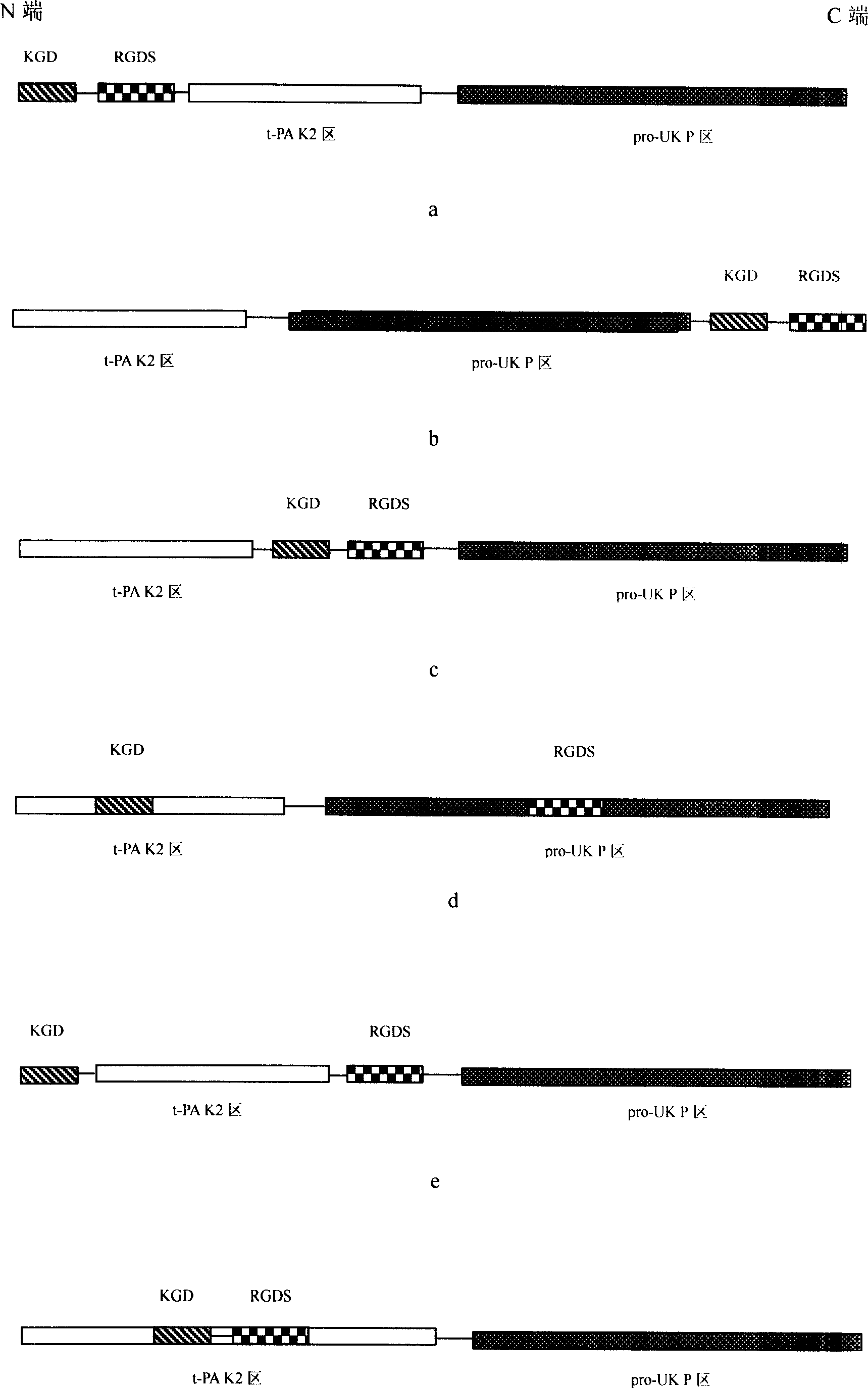
The invention discloses a new abbokinase modifier in the genetic engineering domain, which consists of KGD and RGDS, wherein the tPA K2 segment and abbokinase P area are induced to the N end of molecular structure, which is compatible with platelet aggregation inhibition.
Owner:TIANJIN TASLY PHARMA CO LTD +1
Methods for production of recombinant urokinase
InactiveUS20070009506A1Simple and highly methodSolution stablePeptide/protein ingredientsPeptide preparation methodsRecombinant UrokinasePro-urokinase
Highly efficient methods of producing properly folded recombinant urokinase are provided. Denatured recombinant pro-urokinase is refolded by first solubilizing the protein with a chaotroph at high pH, followed by refolding in the presence of reduced concentrations of chaotroph while the pH is slowly reduced.
Owner:PROTEOMTECH
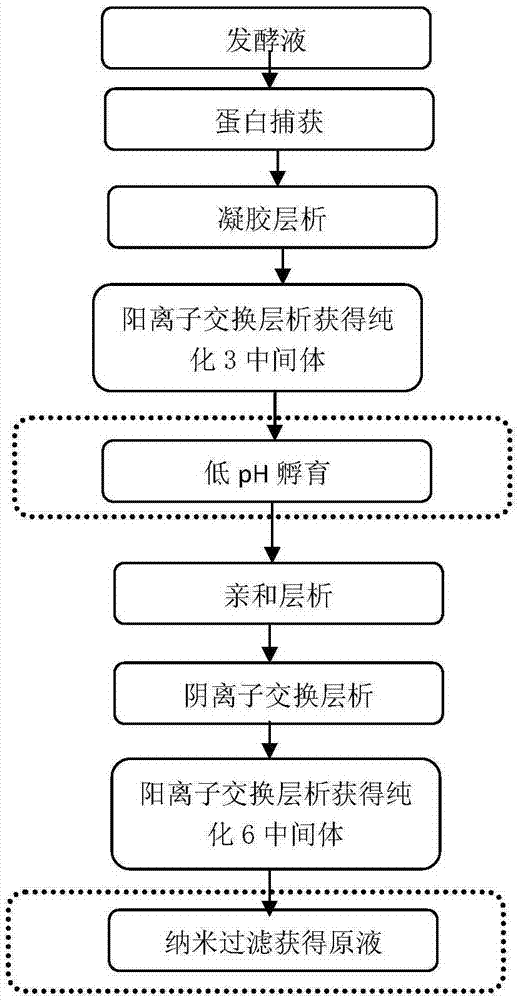
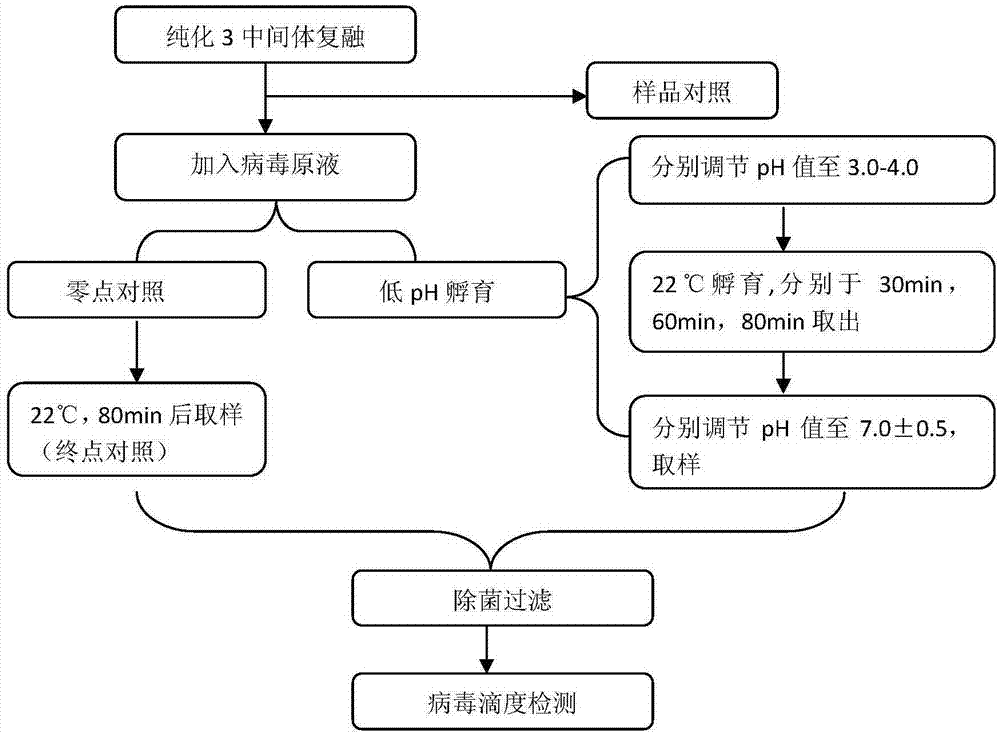
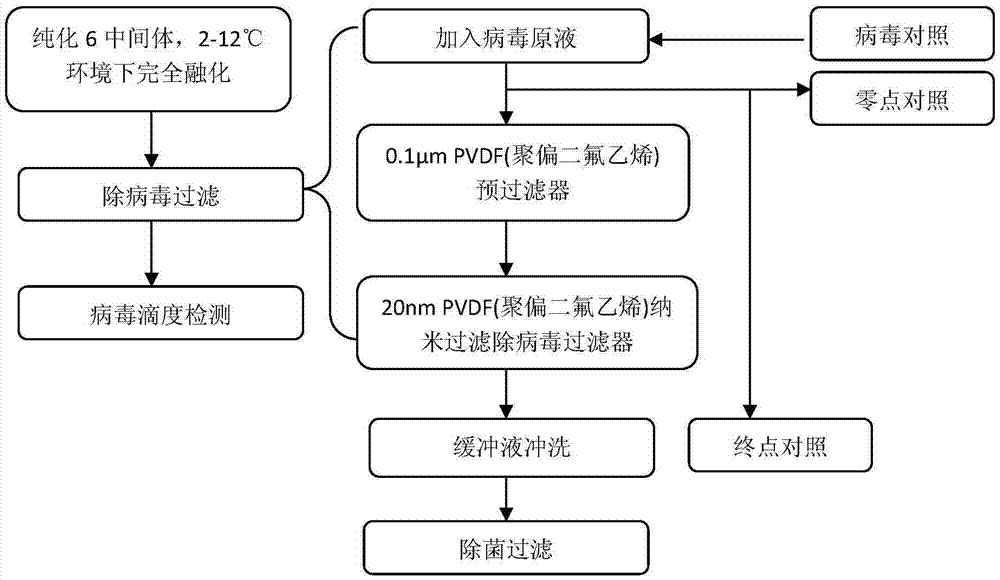
Method for purification and virus removal of recombinant human prourokinase
The invention provides a method for protein purification and virus removal of novel CHO cell-expressed recombinant human prourokinase. The method comprises the steps of protein capture, gel chromatography, cation exchange chromatography, low pH incubation, affinity chromatography, anion exchange chromatography, cation exchange chromatography and nanofiltration. By the adoption of the method, the purity of obtained protein is not lower than 98%, residues of Tris-HCl buffer solution are replaced, and clinical using risks are effectively reduced through virus removal / inactivation.
Owner:TASLY BIOPHARMACEUTICALS CO LTD
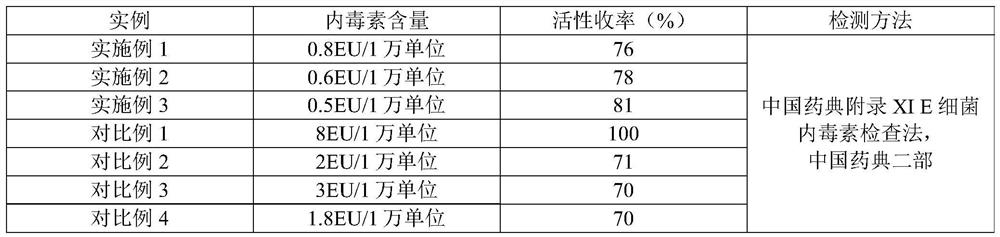
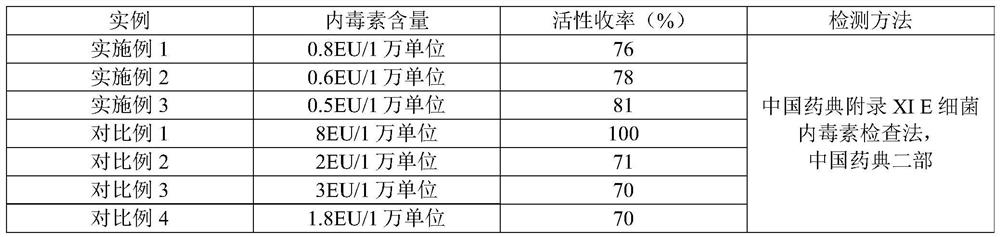
Method for removing endotoxin in human urokinase raw material refining process
ActiveCN111778232ASimple process conditionsEasy to handleIon-exchange column/bed processesPeptidasesEndotoxin removalEngineering
The invention provides a method for removing endotoxin in a human urokinase raw material refining process, and relates to the field of bioengineering and chemical engineering, and the method specifically comprises the following steps: (1) sample pretreatment; (2) preparing and packing of a chromatographic column; and (3) disinfection and sample loading treatment. According to the method, a novel polymer anion chromatography medium is adopted, various process conditions are optimized, the treatment process is simple, endotoxin in a urokinase intermediate product can be effectively removed, andmeanwhile it is guaranteed that the urokinase activity yield reaches 75% or above. In the treatment process, working hours and materials are saved, and the production cost is reduced.
Owner:JIANGSU YOULIKA BIOTECHNOLOYG CO LTD
A kind of purification method of recombinant human urokinase
The present invention provides a purification method of recombinant human prourokinase, including protein capture, gel chromatography, cation exchange chromatography, affinity chromatography, anion exchange chromatography, and cation exchange chromatography: the method increases purification steps, Optimize the purification conditions to increase the protein purity to over 98%.
Owner:TASLY BIOPHARMACEUTICALS CO LTD
A preparation process for separating and purifying recombinant human pro-urokinase from recombinant Escherichia coli fermentation broth
ActiveCN103789291BBig profit marginReduce investmentFermentationVector-based foreign material introductionNatural resourceRecombinant escherichia coli
A preparation process for separating and purifying recombinant human pro-urokinase from recombinant Escherichia coli fermentation broth applied in the technical field of biopharmaceuticals, the preparation process comprises the following steps, using the artificially synthesized human pro-urokinase whole gene sequence as a template, by Obtain the target gene fragment of human prourokinase by PCR, construct a prokaryotic expression vector containing human prourokinase, transform the expression vector into Escherichia coli, screen and pick positive clones, which are genetically engineered strains of human prourokinase; The engineered strain of prourokinase was inoculated into solid LB medium, picked a single colony and inoculated into liquid LB medium, cultured on a shaker at 32°C overnight, then inoculated into the fermentation medium in proportion, and cultivated on a shaker at 32°C overnight, Then inoculate into the fermenter according to the proportion, raise the temperature to 42° C. to induce culture for 4 hours, collect the bacteria by centrifugation, disrupt the bacteria by ultrasonic, and collect the precipitate by centrifugation, which is the inclusion body of human prourokinase. The invention is not limited by natural resources, has the advantages of less investment, high output, large profit margin, unique thrombolytic mechanism, obvious effect, and no or low toxicity and side effects.
Owner:NORTHEAST PHARMA GRP
Composition containing active prourokinase, freeze-drying process and freeze-dried preparation thereof
ActiveCN100420485CFor long-term storageLow pricePowder deliveryPeptide/protein ingredientsFreeze-dryingPro-urokinase
Owner:TASLY BIOPHARMACEUTICALS CO LTD
Method for detecting poloxamer residual quantity in recombinant human urokinase raw material for injection
ActiveCN111983043AReduce contentDifficult to detectComponent separationAgainst vector-borne diseasesMedicinePro-urokinase
The invention relates to a method for detecting poloxamer residual quantity in a recombinant human urokinase raw material for injection. The method comprises the following steps: (1) a sample solutionpreparation method: adding overnight frozen acetonitrile into a raw material sample, oscillating and ultrasonically treating for 2-4 minutes, oscillating for 20-40 seconds, centrifuging at 8000-9800rpm for 1-3 minutes, and taking a supernatant, wherein the ratio of the volume of the original sample to the volume of the acetonitrile is within 1-3; 2) preparing a standard substance solution: taking, weighing and preparing a poloxamer standard substance into standard substance solutions, wherein the concentrations of the standard substance solutions are 2 mg / ml, 1 mg / ml, 0.5 mg / ml, 0.25 mg / ml, 0.1 mg / ml, 0.04 mg / ml, 0.02 mg / ml, 0.01 mg / ml and 0.005 mg / ml in sequence; and 3) detection: respectively taking a standard solution and a sample solution, performing injecting into a high performanceliquid chromatograph, recording a chromatogram detected by CAD, drawing a standard curve by taking the concentration of the standard substance solution as an abscissa and the peak area as an ordinate,calculating the content of poloxamer in the sample solution by using an external standard method, and converting to obtain the content of poloxamer in the recombinant human urokinase raw material forinjection.
Owner:TASLY BIOPHARMACEUTICALS CO LTD
A method for removing endotoxin in the process of refining human urokinase raw materials
ActiveCN111778232BSimple process conditionsEasy to handleIon-exchange column/bed processesPeptidasesEndotoxin removalEngineering
The invention provides a method for removing endotoxin in the process of refining human urokinase raw materials, which relates to the fields of bioengineering and chemical engineering. The method specifically includes the following steps: (1) sample pretreatment; (2) chromatography column preparation and packaging (3) Disinfection and sample loading treatment. The method adopts a new polymer anion chromatographic medium and optimizes various process conditions. The treatment process is simple, the endotoxin in the urokinase intermediate product can be effectively removed, and the urokinase activity yield is guaranteed to be over 75%. In the processing process, man-hours and materials are saved, and production costs are reduced.
Owner:JIANGSU YOULIKA BIOTECHNOLOYG CO LTD
Pharmaceutical composition for ischemic stroke disease as well as preparation method and application of pharmaceutical composition
PendingCN113855790AInhibition of exudationInhibit cerebral hemorrhagePowder deliveryOrganic active ingredientsDiseaseMedicine
The invention relates to a pharmaceutical composition for an ischemic stroke disease as well as a preparation method and application of the pharmaceutical composition. The pharmaceutical composition mainly comprises salvianolic acid and human recombinant human pro-urokinase. The invention further relates to application of a combination of the salvianolic acid and the human recombinant human pro-urokinase in preparation of a medicine for preventing or treating ischemic stroke. The pharmaceutical composition disclosed by the invention can be used for preventing or treating the ischemic stroke and meanwhile reducing bleeding or bleeding tendency of a patient.
Owner:TIANJIN TASLY ZHIJIAO PHARMA
Freeze-drying method of recombinant human pro-urokinase for injection
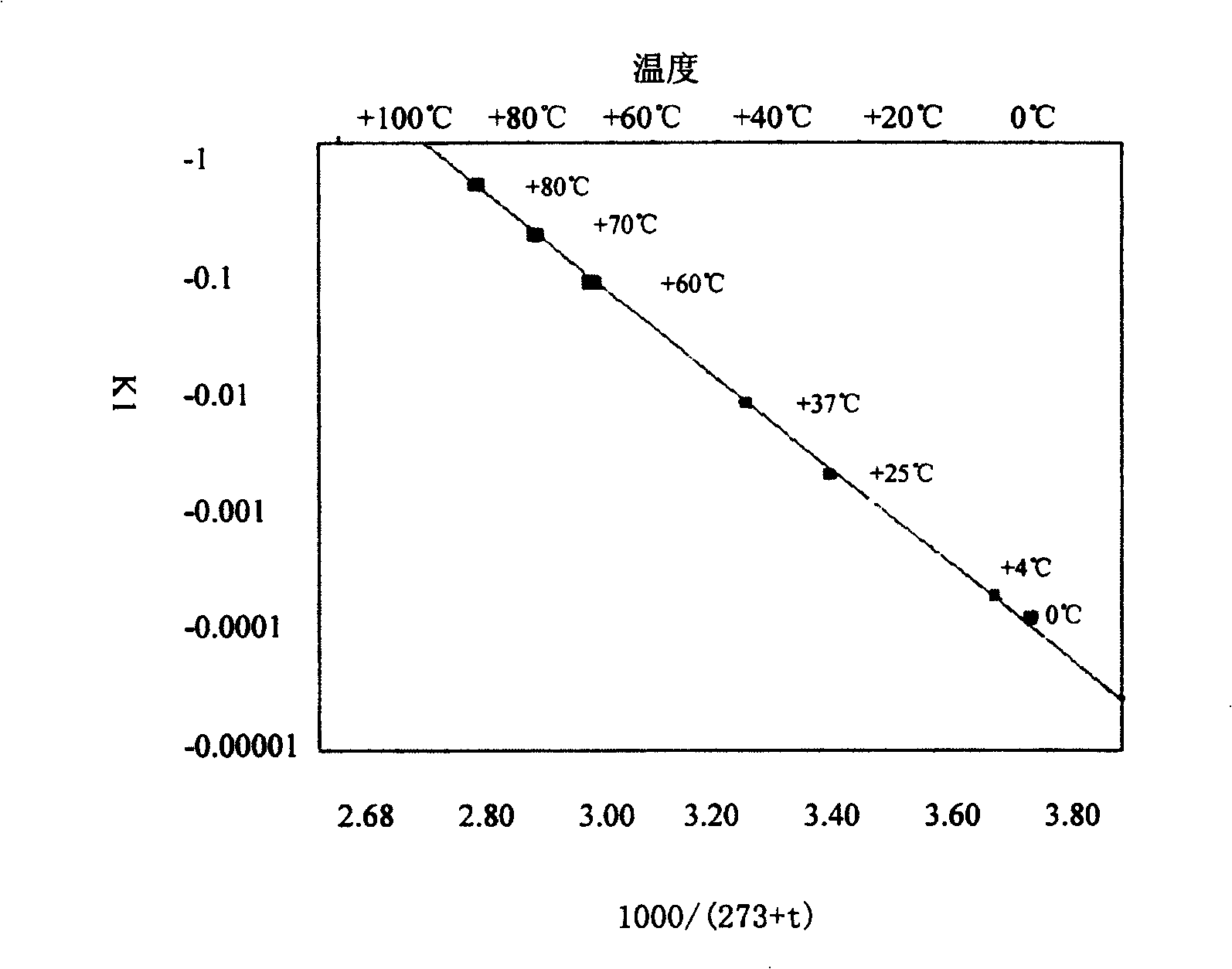
PendingCN113116831AEasy to collapseChange shapePowder deliveryPeptide/protein ingredientsPenicillinPhysical chemistry
The invention relates to a freeze-drying method of recombinant human pro-urokinase for injection, which comprises the following steps: 1) pre-freezing and annealing of a product, mixing raw materials and auxiliary materials and subpackaging into penicillin bottles, then putting into a freeze-drying box for pre-freezing the product, setting the temperature of a plate layer to be-38 DEG C to-42 DEG C in the pre-freezing process, keeping for 50-70 minutes, and annealing at-22 to-28 DEG C after the temperature of the product is stable; 2) primary drying: a freeze-drying box body needs to be aerified, the aerification is controlled to be 20 + / -5 Pa, and the temperature of a plate layer is raised to-20 DEG C to-10 DEG C and is kept stable until the waterline of a product disappears; and 3) secondary drying (desorption drying): after the primary drying is finished, the temperature of the plate layer is raised to 30-40 DEG C, secondary drying is carried out, and the drying temperature is controlled to be 35 + / -5 DEG C.
Owner:TASLY BIOPHARMACEUTICALS CO LTD
Recombinant human prourokinase preparation for injection as well as preparation method and application thereof
PendingCN114668715AStable structureEasy to degradePowder deliveryPeptide/protein ingredientsActive agentPro-urokinase
The invention relates to a recombinant human pro-urokinase preparation for injection as well as a preparation method and application thereof, the recombinant human pro-urokinase preparation for injection is a liquid preparation, the formula of the recombinant human pro-urokinase preparation comprises recombinant human pro-urokinase, a protective agent, a surfactant and buffer salt, and a solvent is water for injection. According to the recombinant human pro-urokinase preparation for injection, the protective agent and the surfactant are used in a combined mode and jointly serve as the protective agent of the recombinant human pro-urokinase, so that the structure of the recombinant human pro-urokinase is more stable, and the recombinant human pro-urokinase is not prone to degradation. The invention further provides a combined suite containing the recombinant human prourokinase freeze-drying preparation for injection, the combined suite comprises the recombinant human prourokinase freeze-drying preparation for injection, normal saline, a quick connection communicating vessel and an injector which are independently packaged, and compared with the prior art, the combined suite has the advantages that the working efficiency of medical staff can be improved, the compliance is improved, the rescue time can be saved, and the safety of medical staff is improved. And the use experience of medical staff is improved, and good practicability and practical significance are achieved.
Owner:TASLY BIOPHARMACEUTICALS CO LTD
Manufacturing method of adjustable liver damage animal model and special DNA fragment thereof
The invention discloses a manufacturing method of an adjustable liver damage animal model and a special DNA fragment thereof; a DNA fragment I is formed by sequentially connecting liver super promoters and antisense tetracycline activator coding genes of a Tet-on gene expression system from upstream to downstream; an DNI fragment II is formed by sequentially connecting tetracycline reaction factors, promoters and prourokinase activator coding genes of the Tet-off / Tet-on gene expression system from upstream to downstream; a recombination expression vector carrying the DNI fragment II is used for converting mammalian cells, the cells are cultivated to obtain recombinant adenovirus. The DNA fragment I is led in the animal to obtain a transgenosis first-construction animal; the transgenosis first-construction animal and scid / bg animal are back-crossed to obtain the animal carrying the DNA fragment I with the scid / bg background; the recombinant adenovirus is used for injecting the animal carrying the DNA fragment I with the scid / bg background, so as to obtain the adjustable liver damage animal model; in the model, the prourokinase activator expression is activated by doxycycline, the expression intensity is changed along with the change of dosage, the specificity is very high.
Owner:PEKING UNIV
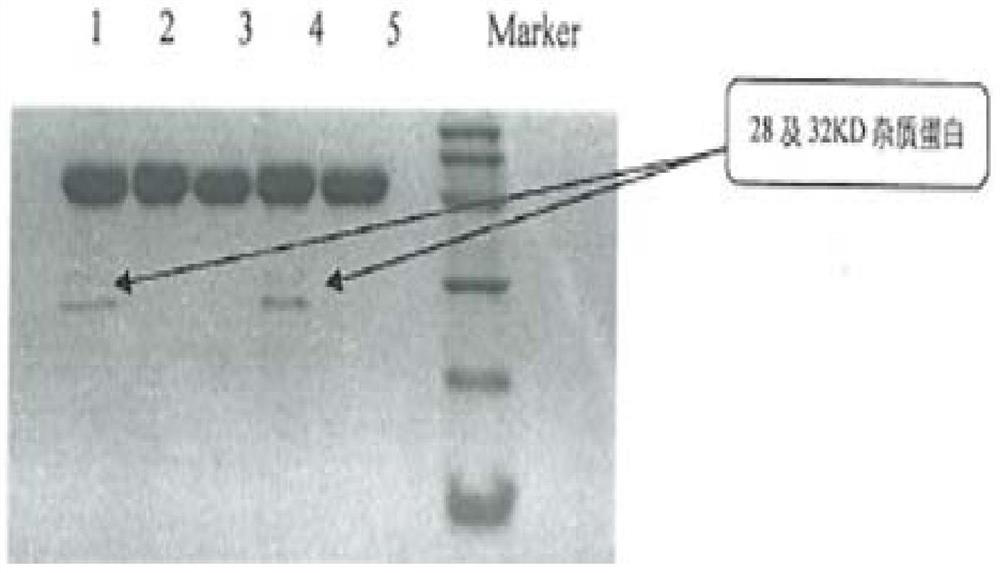
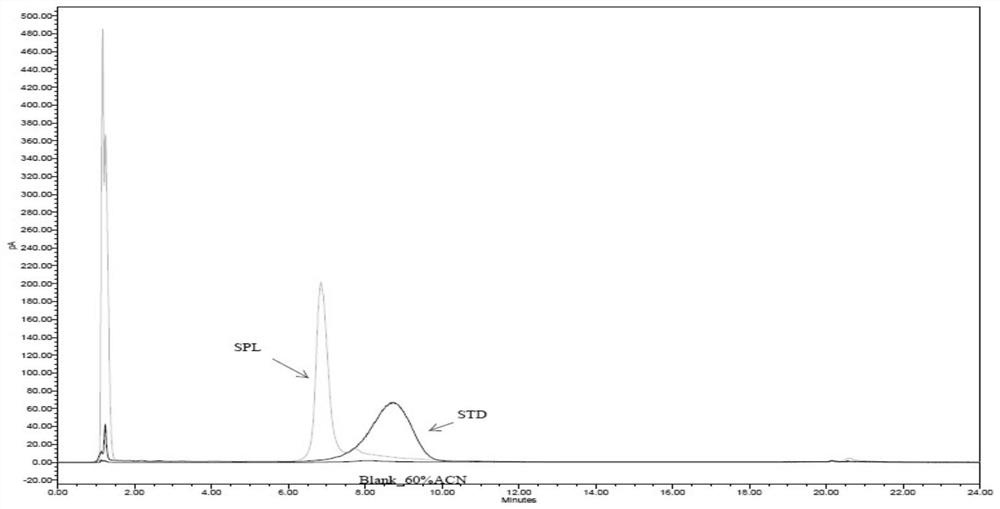
Capillary electrophoresis detection method for analyzing impurities of recombinant human prourokinase for injection and application of capillary electrophoresis detection method
PendingCN114689674AEfficient separationEasy to distinguishMaterial analysis by electric/magnetic meansElectrophoresesCapillary electrophoresis
The invention discloses a capillary electrophoresis detection method for analyzing impurities of recombinant human pro-urokinase for injection and application of the capillary electrophoresis detection method. The capillary electrophoresis detection method comprises the following steps: (1) diluting a sample which comprises a non-reducing sample or a reducing sample by using a sample buffer solution, then adding a reducing agent into the reducing sample or adding an alkylating reagent into the non-reducing sample, and mixing; (2) heating a sample obtained by mixing in the step (1), cooling, centrifuging, and transferring into a loading tube; and (3) placing the sample loading tube on a capillary electrophoresis apparatus, operating the capillary electrophoresis apparatus, starting to inject the sample, separating the sample to obtain a capillary electrophoresis detection map, and analyzing the map to obtain a detection result. According to the method, impurities in the recombinant human pro-urokinase can be effectively separated, the reproducibility of multiple batches of samples is good, and the accuracy is high.
Owner:TASLY BIOPHARMACEUTICALS CO LTD
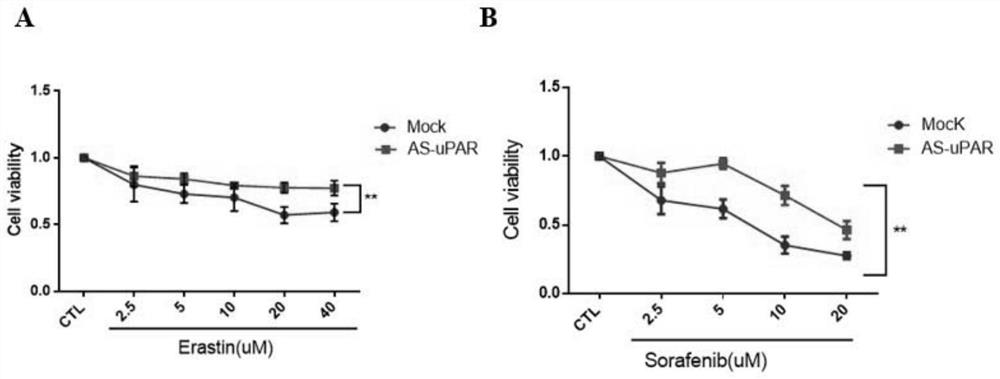
Method for enhancing sensitivity of tumors to drug
InactiveCN112057633AIncreased sensitivityOrganic active ingredientsGenetic material ingredientsHuman tumorCell-Extracellular Matrix
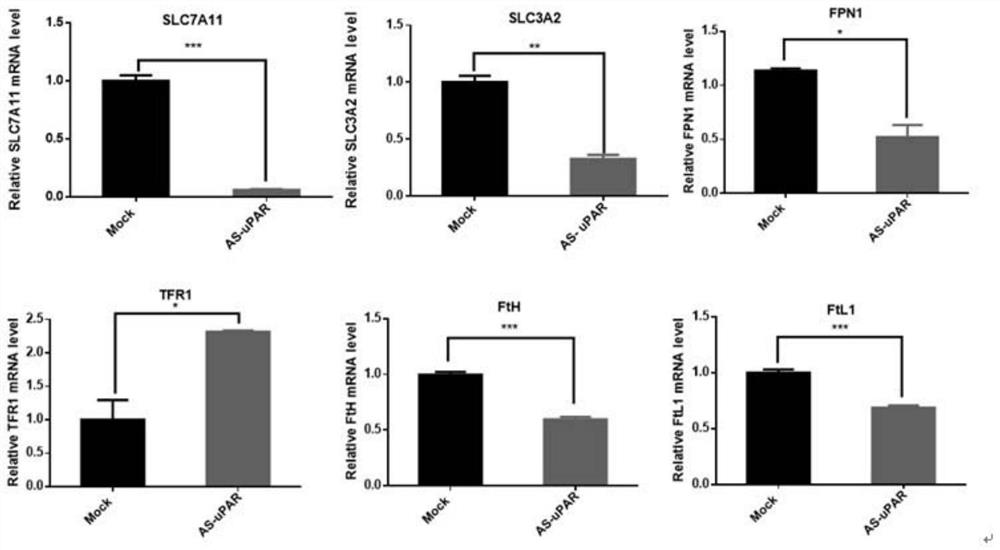
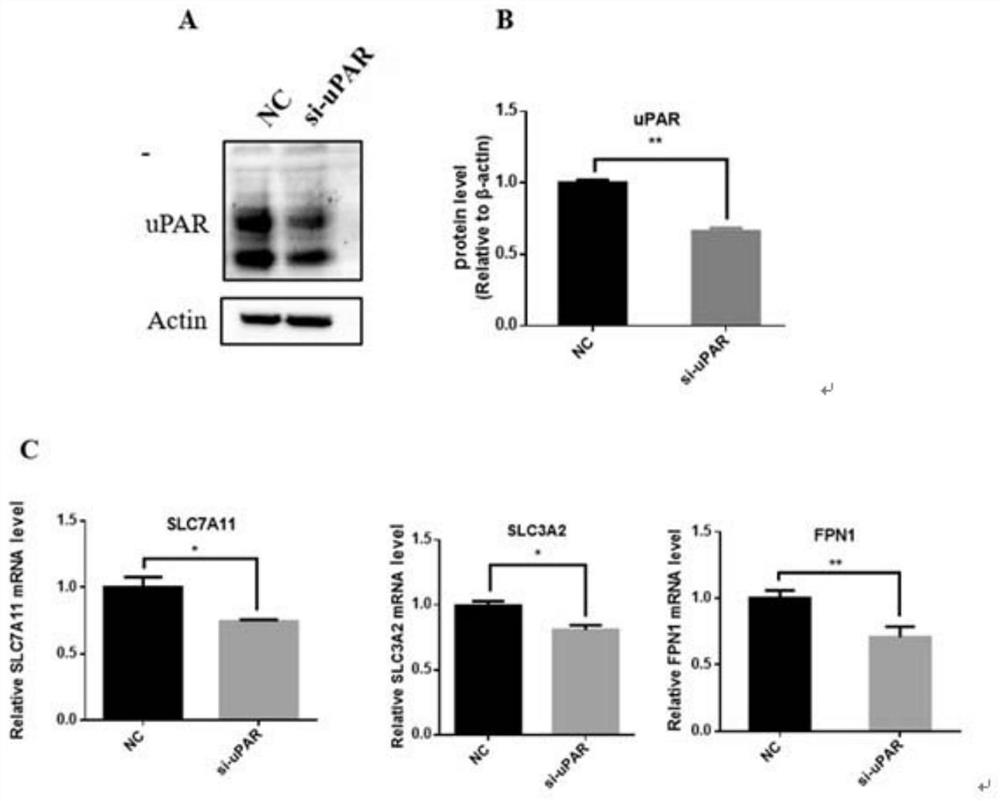
The invention discloses a method for enhancingthe sensitivity of tumors to a drug. The method comprises the following step: the sensitivity of human tumor cells to the drug is enhanced by down-regulating expression of a urokinasetype plasrinogen activator receptor (uPAR). The expression of the uPAR is inhibited through RNA interference and antisense nucleic acid, so that the sensitivity of the tumor cells to the drug is enhanced. According to the method, the sensitivity of the tumor cells to drug therapy is enhanced by regulating and controlling ferroptosis through the uPAR. The sensitivity ofthe tumor cells to drug therapy is enhanced by regulating and controlling ferroptosis through the uPAR, and thus the uPAR for regulating and controlling tumor extracellular matrix is linked with ferroptosis.
Owner:NANJING JIRUIKANG BIOTECHNOLOGY RES INST CO LTD
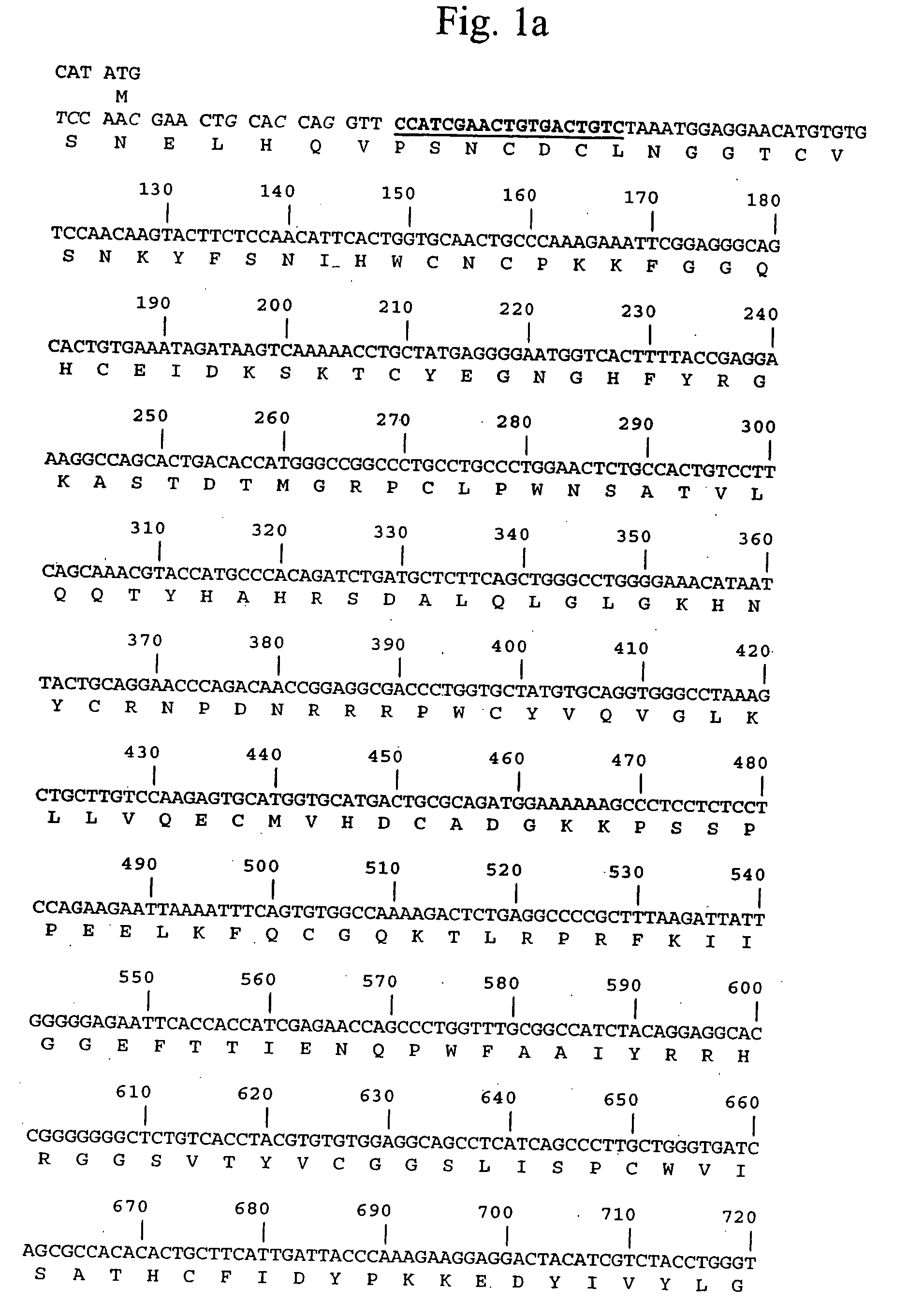
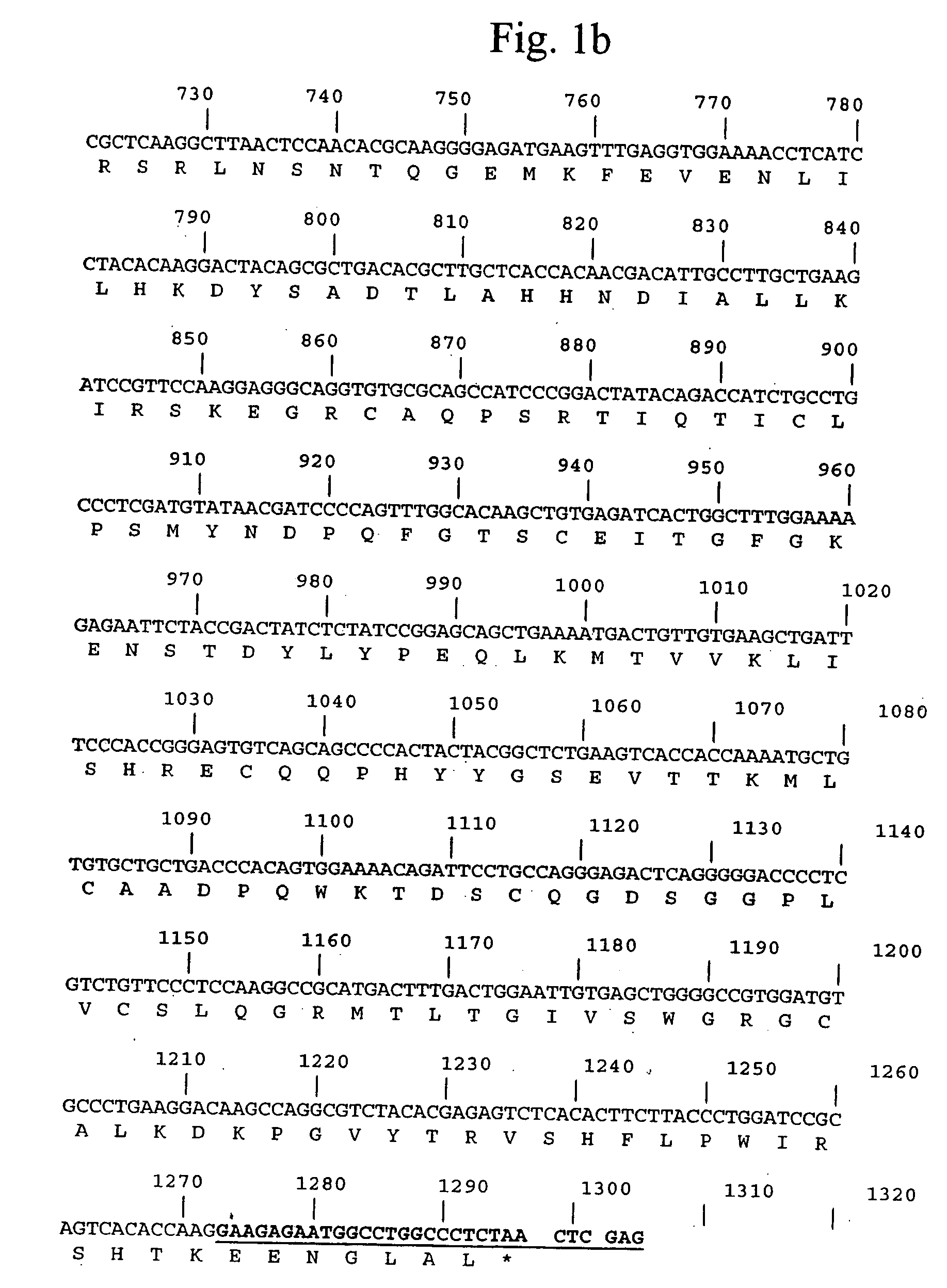
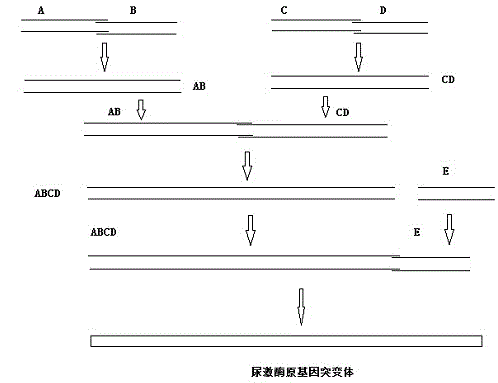
Pro-urokinase gene mutant and preparation method thereof
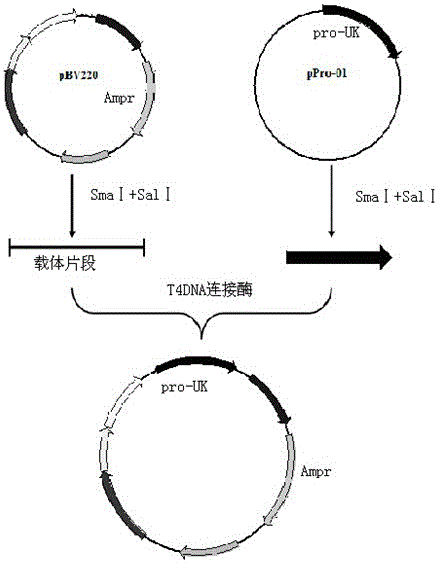
InactiveCN103146727AOptimize the genetic codeEasy constructionFermentationPlant genotype modificationSequence analysisEscherichia coli
The invention relates to a pro-urokinase gene mutant and a preparation method thereof. On the premise of keeping the amino acid composition and sequence of a protein unchanged, two nucleotides G and T are added on the 5' end of the gene sequence, and a recognition sequence GTCGAC of endonuclease Sal1 is added on the 3' end of the sequence. The nucleotide sequence of the gene mutant is shown in a sequence table 1. The preparation method comprises the following steps: (1) synthesizing a single-chain nucleotide segment; (2) performing PCR (polymerase chain reaction) to generate a double-chain DNA (deoxyribonucleic acid) segment; (3) splicing the whole sequence of the pro-urokinase gene mutant; and (4) determining and analyzing the sequence of the pro-urokinase gene mutant. Through a method of artificially synthesizing the whole gene, the invention optimizes the whole genetic codon, appropriately modifies both ends of the gene and ensures that sequencing analysis, expression vector construction and other further genetic manipulations can be easily performed. Thus, the invention develops a novel pro-urokinase gene suitable for being expressed in Escherichia coli, so that pro-urokinase can be conveniently produced through an Escherichia coli fermentation technology.
Owner:NANCHANG WANHUA BIOCHEM PHARMA
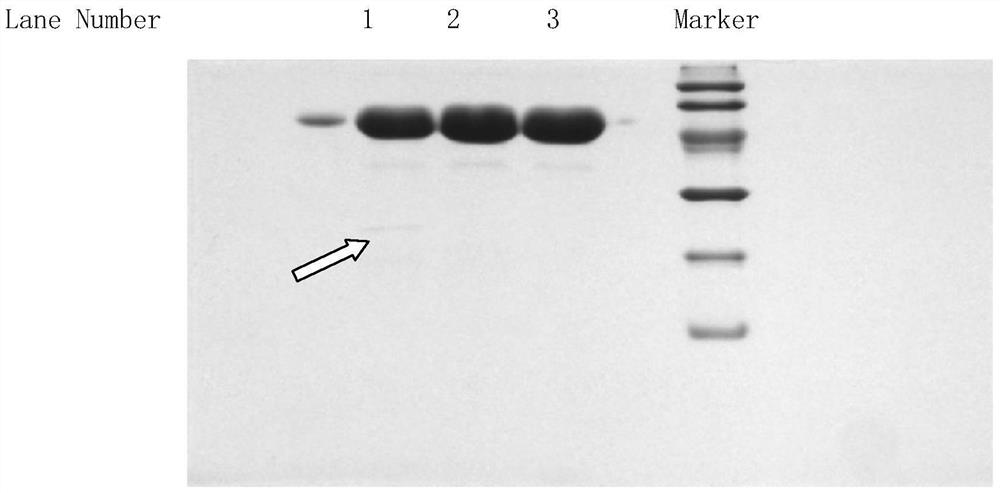
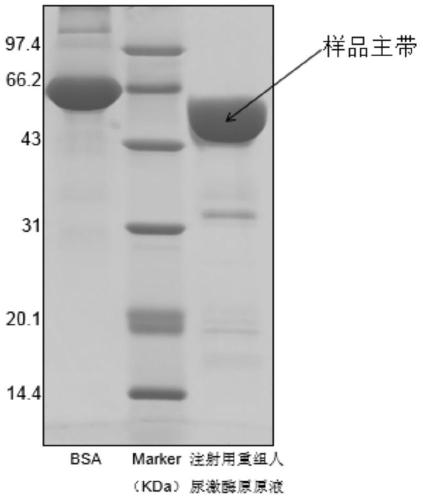
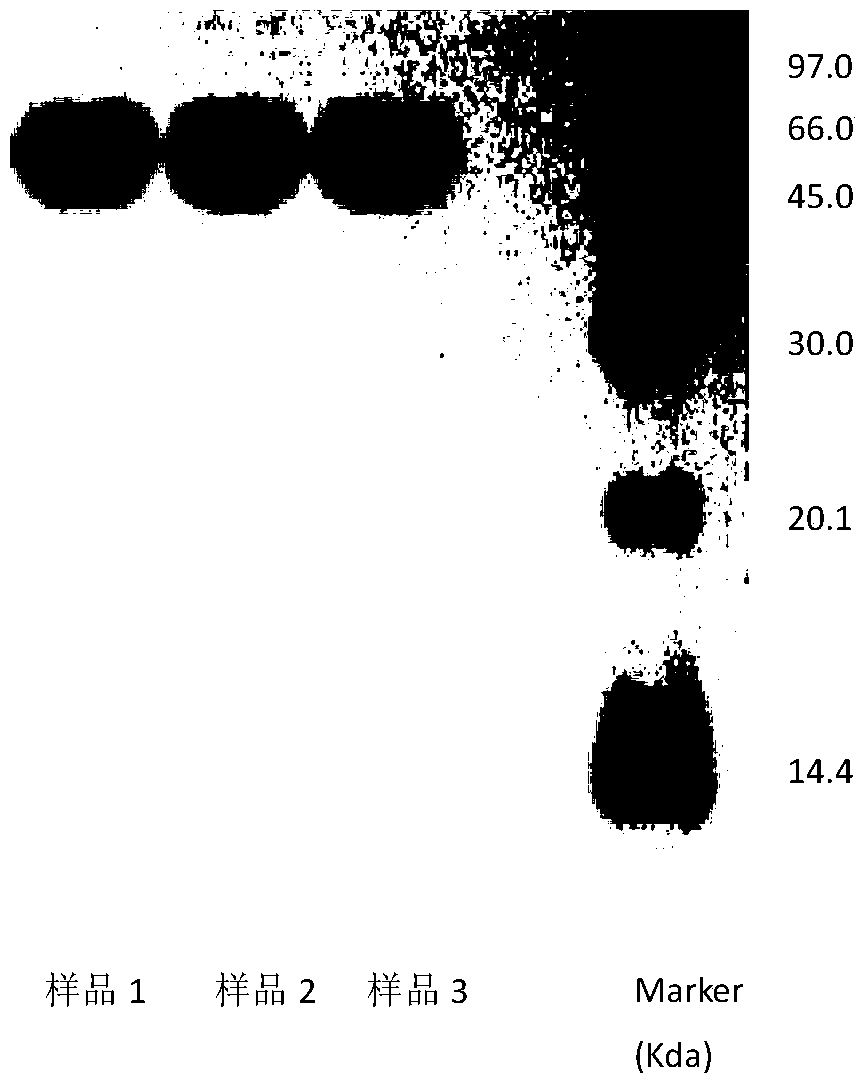
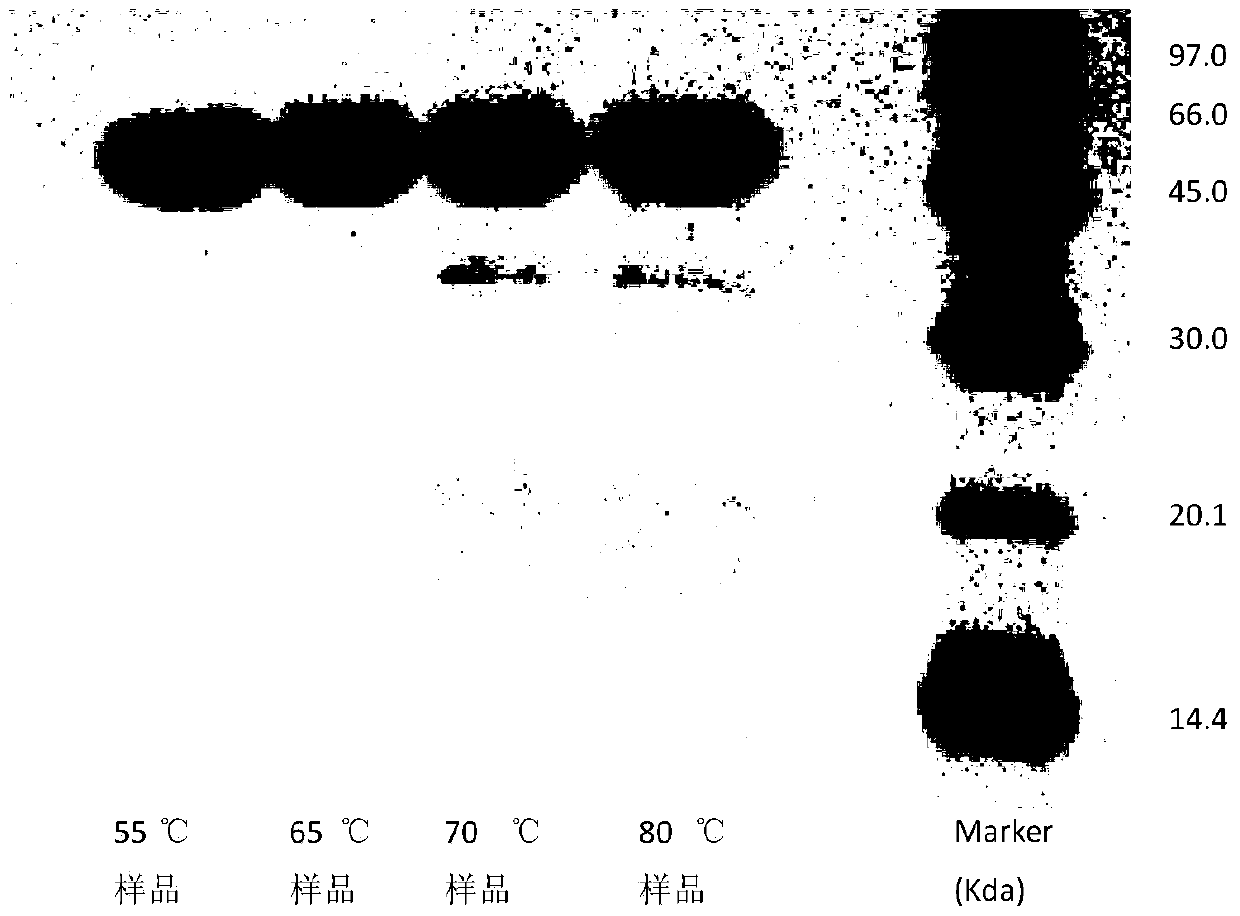
A method for determining the electrophoretic purity of recombinant human prourokinase for injection
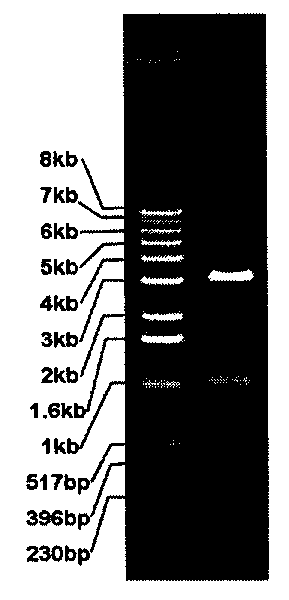
The invention relates to a method for electrophoresis determination of purity of recombinant human prourokinase for injection, wherein the method includes the following steps: (1) preparation of a test sample solution: taking the recombinant human prourokinase for injection on ice, thawing, diluting with MilliQ water, adding a non reductive sample buffer solution, placing at the temperature of 55-65 DEG C and heating for 1-5 minutes, immediately placing in an ice bath to obtain the non reductive test sample solution; (2) preparation of a Marker solution: taking a Marker solution, thawing, placing in an 80-100 DEG C water bath for 1-5 minutes, and immediately placing in an ice bath for standby application; and (3) determination method: adopting a vertical plate gel electrophoresis system, and detecting the test sample solution and the Marker solution, wherein the sample loading amount of the two solutions is 10-20 [mu]L, and the instrument initial voltage is 50-60 V; and adjusting the voltage to 100-110 V when entering a separation gel, when a bromophenol blue band runs to the lower edge of the gel, finishing the electrophoresis, after electrophoresis, dyeing and decoloring to obtain an atlas, and calculating the purity of the test sample solution according to the atlas.
Owner:TASLY BIOPHARMACEUTICALS CO LTD
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com