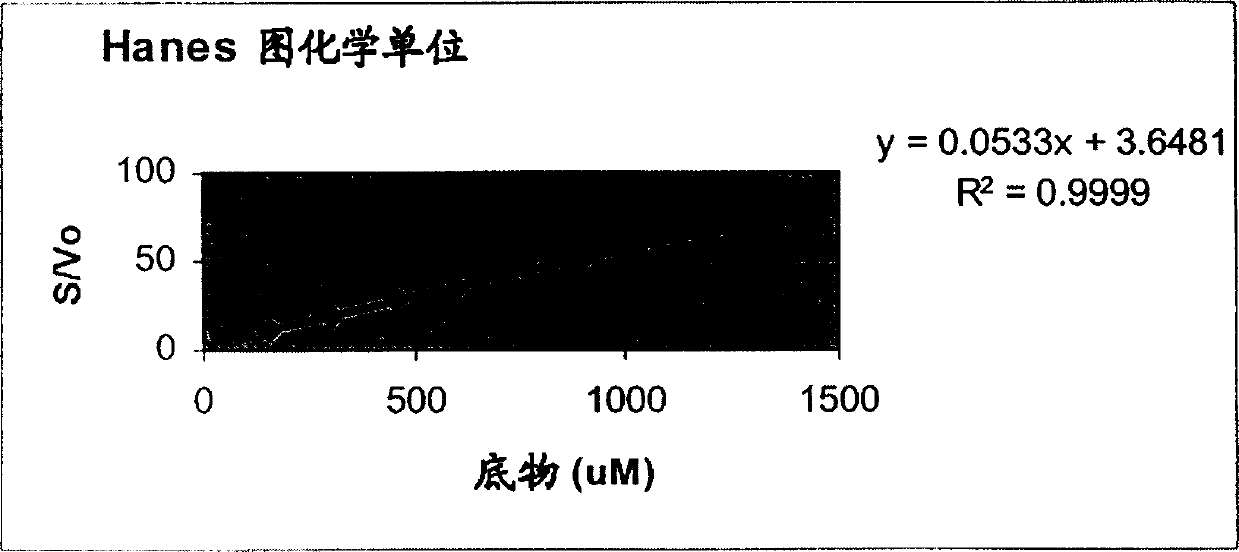
Method for producing recombinaton urokinase
A technology of prourokinase and urea, applied in the field of production of recombinant urokinase, which can solve problems such as safety and reproducibility concerns
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0061] Example 1: Refolding and purification of recombinant prourokinase
[0062] The kidney cDNA library was amplified by PCR to generate a DNA fragment encoding human prourokinase. The primers used in the above PCR reaction were UK-1 (5'-CATATGTCCAACGAACTGCACCAGGTTCCATCGAACTGTGACTGTC-3'[SEQ ID NO: 3]) and UK-2(5'- CTCGAGTTAGAGGGCCAGGCCATTCTCTTC-3' [SEQ ID NO: 4]). Primer UK-1 was used to introduce 6 silent mutations into the prourokinase gene, which can increase the expression efficiency in E. coli.
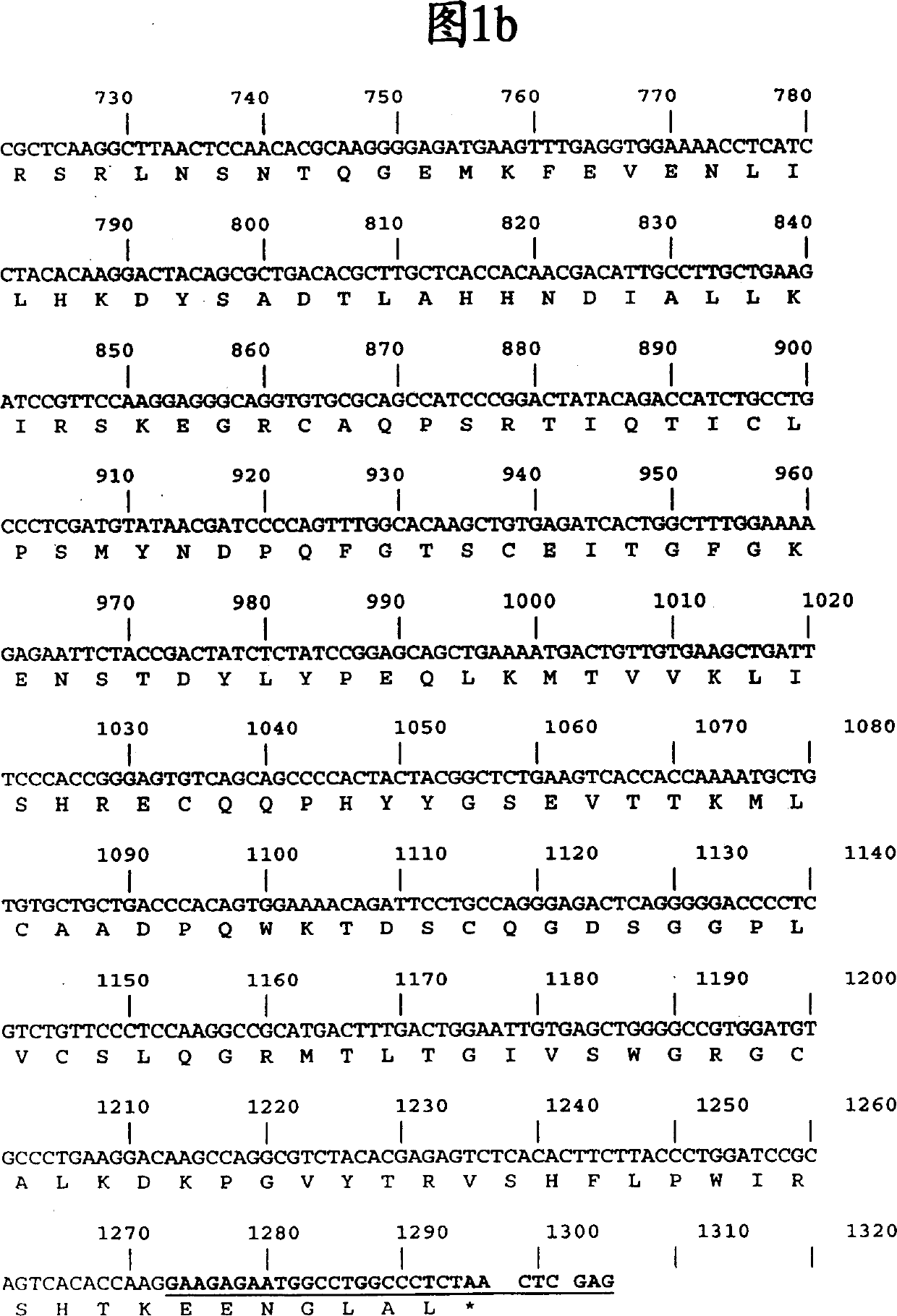
[0063] The full-length PCR product was cloned into pCR2.1 TOPO (Invitrogen) and sequenced from both ends using M13F and M13R primers. Figure 1 shows the nucleotide and its encoded protein sequence. The insert was excised using NdeI-XhoI restriction digestion, gel purified, and cloned into NdeI-XhoI digested pET43 (Novagen).
[0064] The pro-urokinase expression vector was transformed into Escherichia coli BL21 (DE3) strain and spread on the ZB plate containing ampicillin. ...
Embodiment 2
[0072] Example 2: Purification of recombinant prourokinase by heparin affinity chromatography and hydroxyapatite chromatography
[0073] The refolded pro-urokinase produced as described in Example 1 was concentrated by ultrafiltration (MilliporePellicon, 10,000Da cut-off membrane), and then subjected to SEC. A SEPHACRYL® S-300 column equilibrated with 1 mM EDTA, pH 8.0. Refolded prourokinase is separated from misfolded high molecular weight aggregates by this chromatography.
[0074] The urokinase-containing fraction was collected from the S-300 column, diluted 5-fold with 25 mM HEPES pH 7.0, and loaded into HiTrap TM Heparin HP agarose affinity chromatography column (Pharmacia / Amersham). The heparin affinity chromatography column was first equilibrated with 25mM HEPES pH 7.0, 25mM NaCl. After the sample is loaded on the column, wash the column with equilibration buffer until no protein is detected in the effluent. Thereafter, the chromatographic column was washed with ...
Embodiment 3
[0075] Example 3: Identification of recombinant urokinase
[0076] The purified human prourokinase produced according to Example 1 was diluted to 1 μM with 50 mM tris, 50 mM NaCl, 0.01% TWEEN® 20, pH 8.9, and incubated with plasmin (0.1 μg / mL) at 37° C. 60 minutes to convert to urokinase. The reaction was terminated by adding excess (12,500 IU / mL) aprotinin.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com