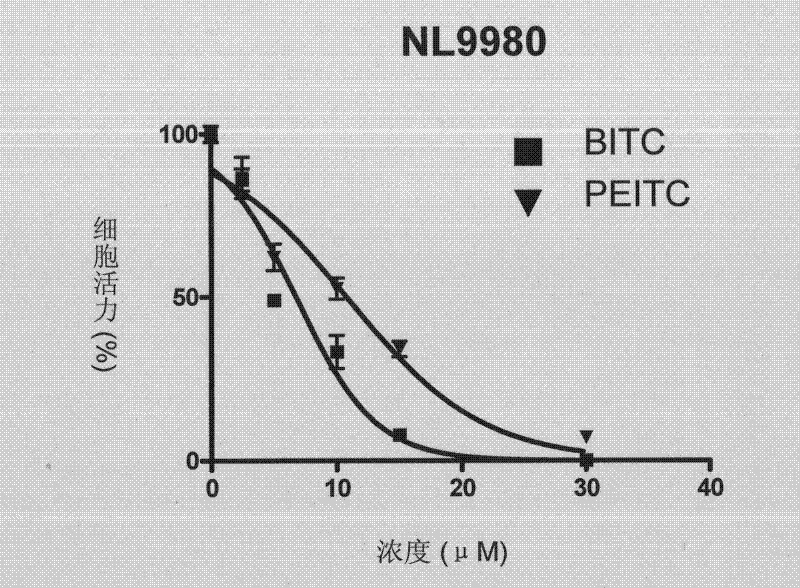
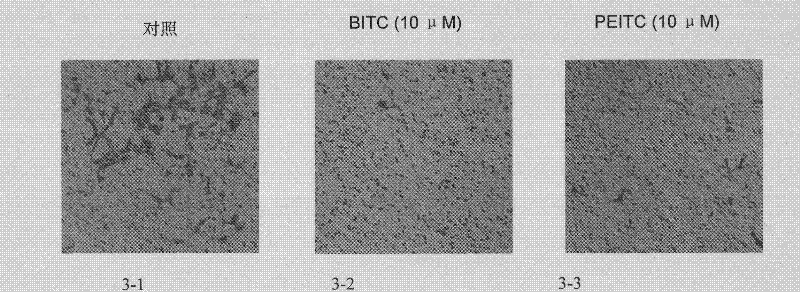
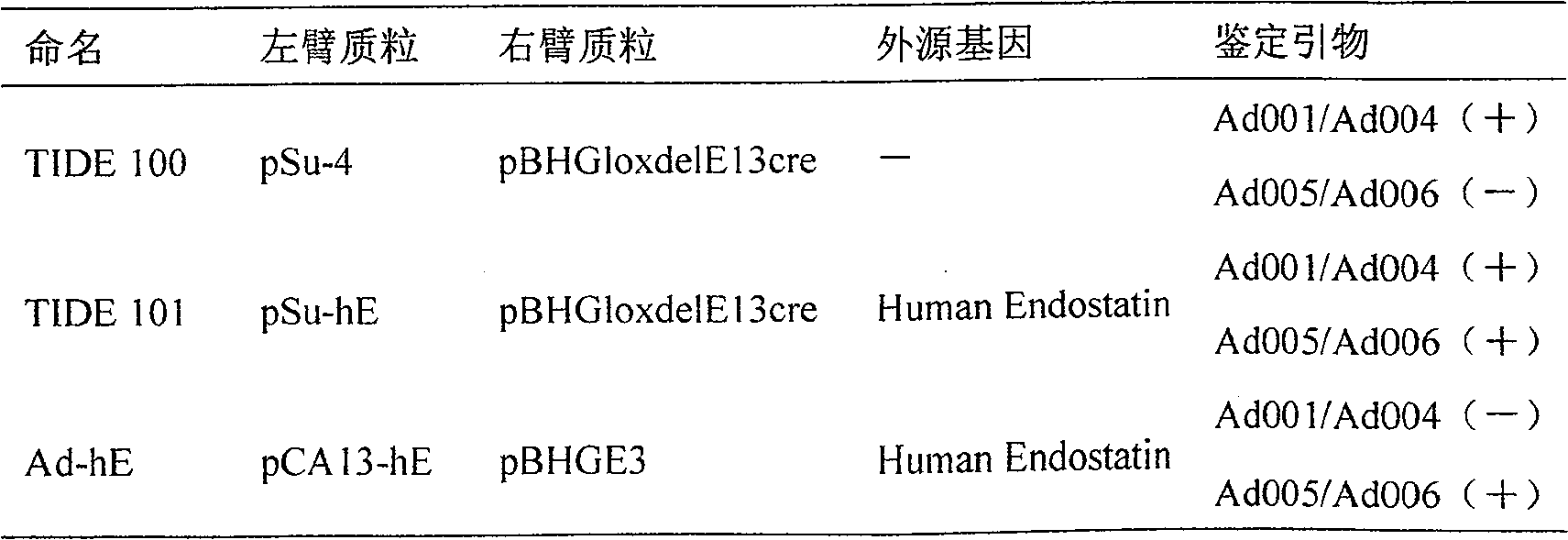
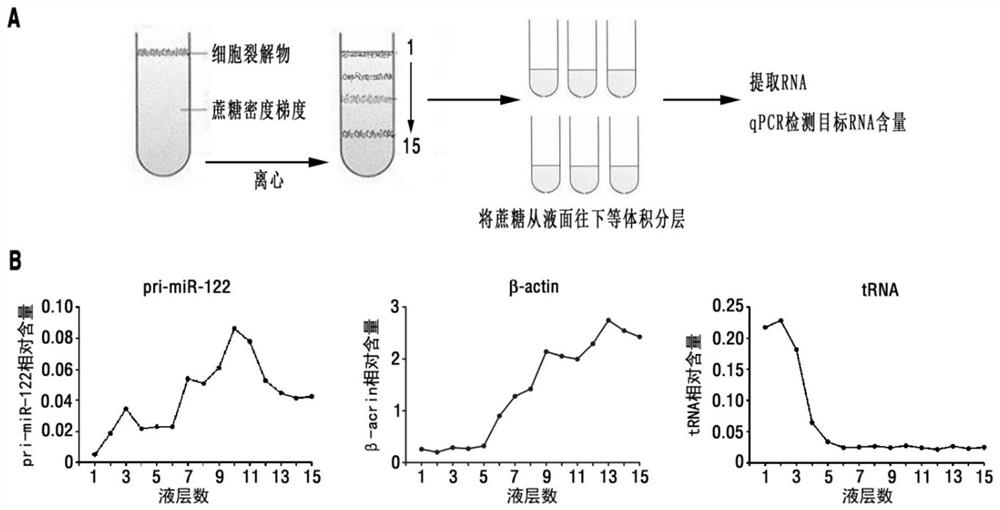
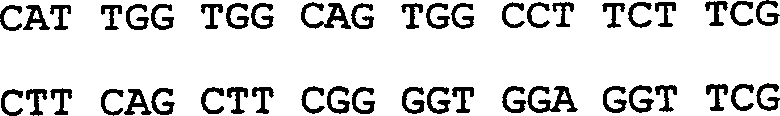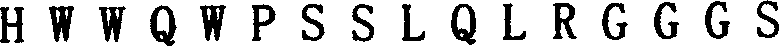Patents
Literature
52results about How to "Inhibition of invasion and metastasis" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
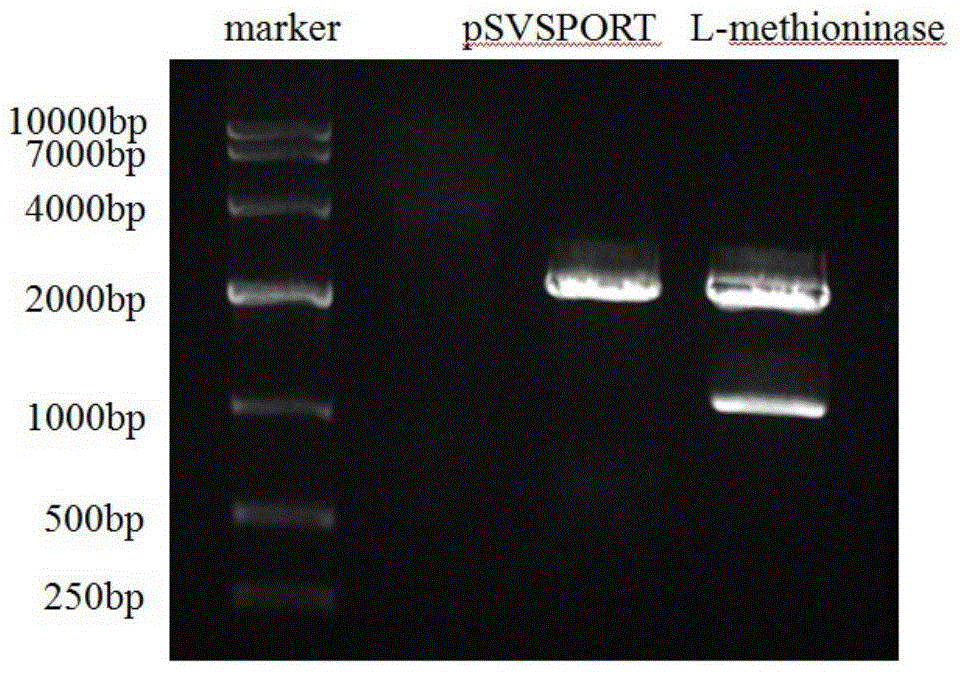
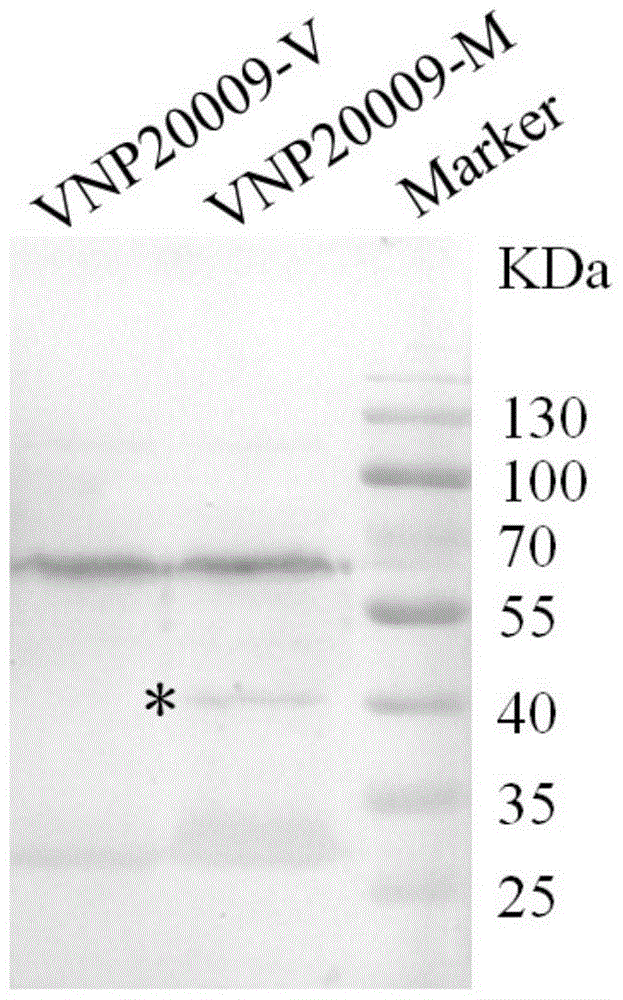
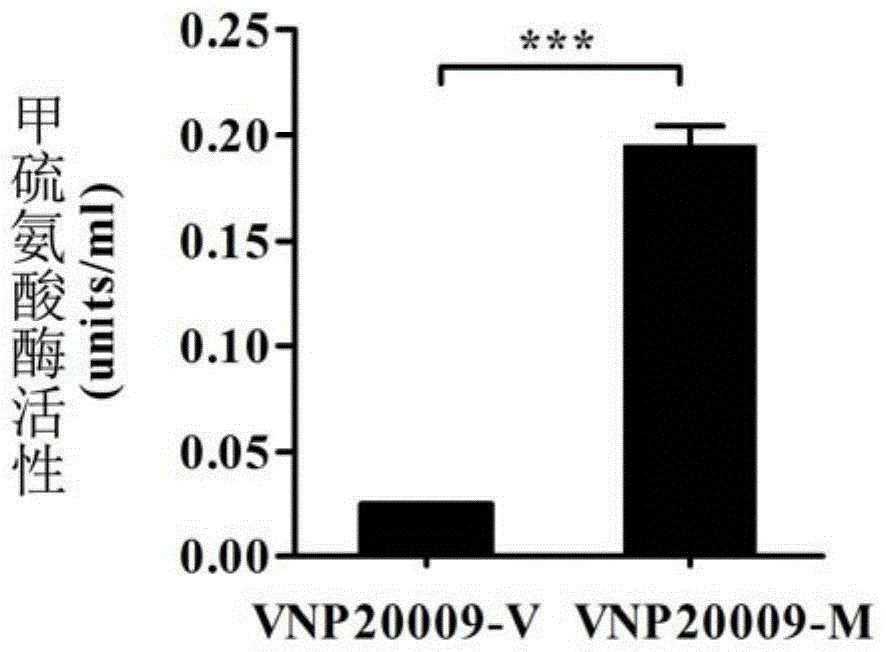
Application of genetically engineered bacterium VNP-20009-M in preparation of medicines for preventing and treating cancer metastasis
PendingCN105983103AInhibit transferHas antitumor effectCarbon-sulfur lyasesBacteriaTumor targetCancer cell
The invention discloses application of genetically engineered bacterium VNP-20009-M of attenuated salmonella typhimurium in preparation of medicines for preventing and treating metastasized cancers. The genetically engineered bacterium VNP20009-M has a tumor targeting property on cancer cells, has a remarkable effect for inhibiting metastasis and growth of cancer cells, and can be used for preparing medicines for preventing and treating metastasized tumors.
Owner:GUANGZHOU SINOGEN PHARMA CO LTD
Tumour-dissolving adenovirus mutant possessing multiple specific anti-tumour mechanism
InactiveCN1884556AGood curative effectPlay a therapeutic roleFermentationGenetic engineeringHuman tumorReverse transcriptase
This invention involved oncolytic adenovirus mutant with the multiple antitumoral Mec. It belongs to the BME field. The adenovirus mutant Elb-55kDa and Elb-19kDa has missing gene, inserts chimeric promoter composed by the human telomerase reverse transcriptase core sequence and human tumor epidermal growth factor receptor enhancer before the replication required gene Ela codons mEla289R and mEla243R. So the virus can specific proliferate in cancer cell. Oncolytic viruses and mEla protein can exert thire influence. It can also increase the sensitivity of cancer cell to chemoradiation and have no influence to normal cell. It inserts the human h-endostatin gene expression cassette in adenovirus mutant gene group; along the replication of virus in cancer cell to amplificate h-endostatin gene and effective expression in tumor so to restrain neovascularization of tumor so to realize the result of restrain the increase and transformation of cancer cell and apoptotic cell. This new oncolytic adenovirus mutant has good clinical prospect in gene curing so to used in curing many kinds of human tumors.
Owner:JIANGSU SHUNTANG BIOENG
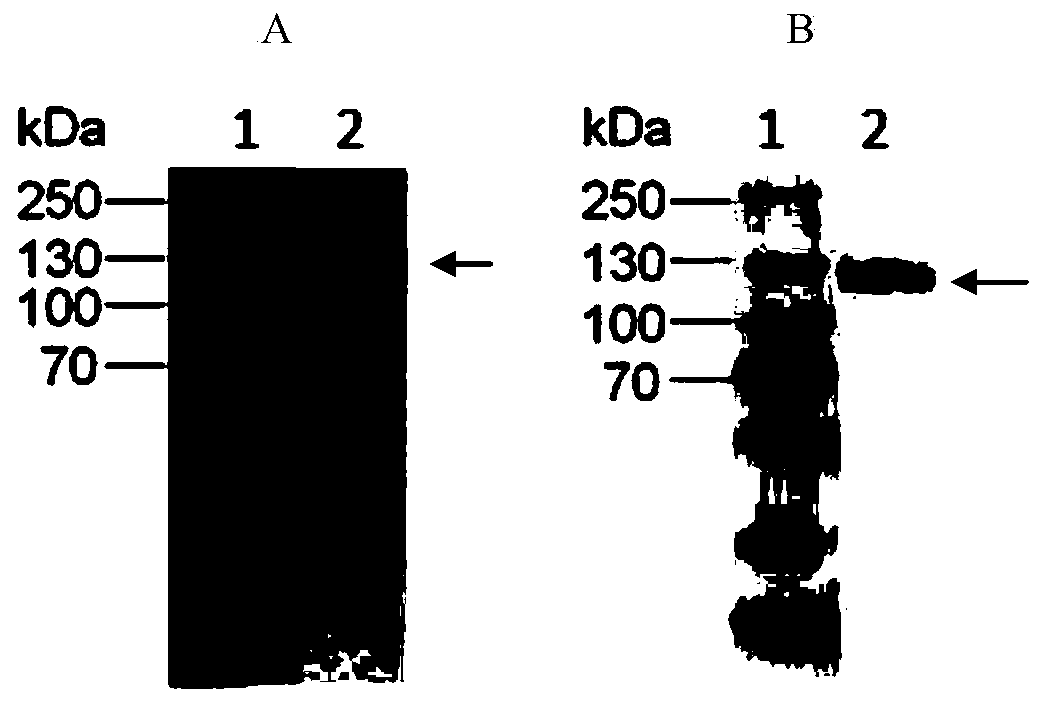
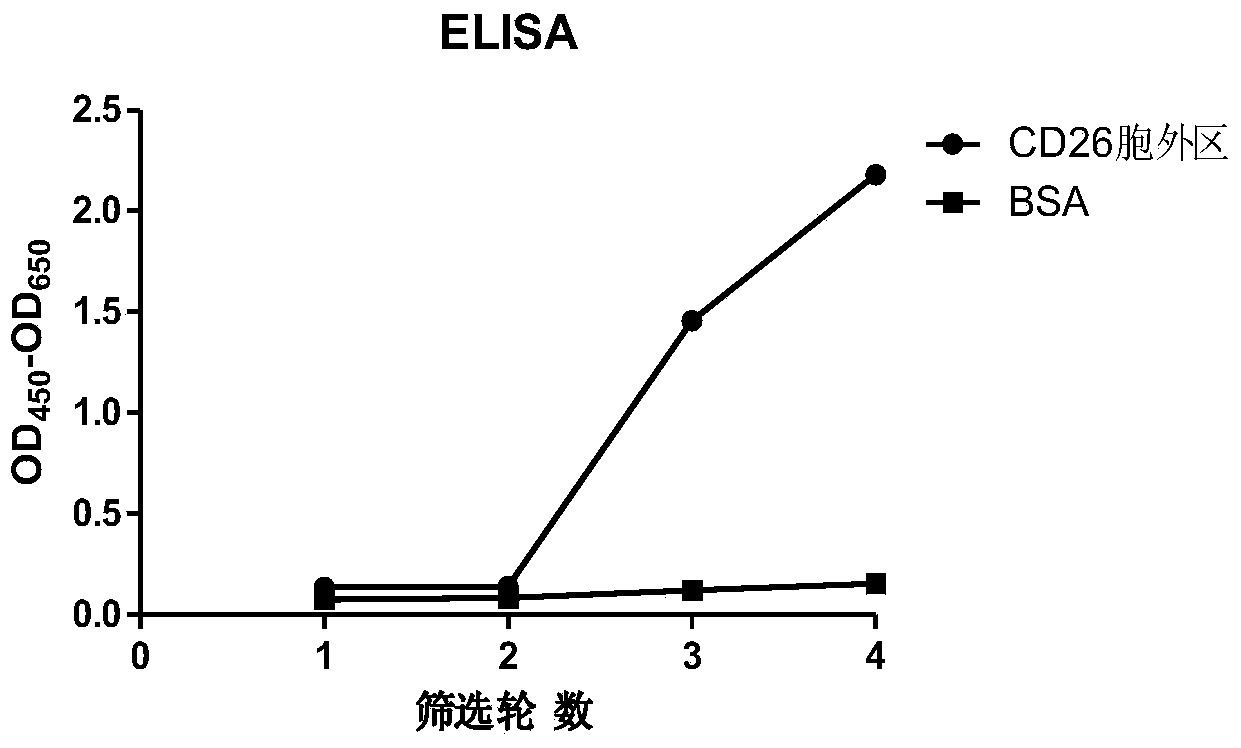
Humanized anti-CD26 antibody and application thereof
ActiveCN103724431APrevent proliferationPrevent invasionFungiBacteriaSingle-Chain AntibodiesComplementarity determining region
The invention provides a novel high-affinity completely humanized antibody which can specifically bind with CD26 as well as a preparation method and application thereof and belongs to the technical field of genetic engineering antibodies. The CD26 is a ubiquitous multifunctional II type transmembrane glycoprotein, has various biological functions and can be interacted with various proteins such as ADA, CD45, FAP-alpha and the like. The invention provides the humanized antibody or a fragment thereof, wherein the antibody or the fragment thereof is capable of specifically binding with the human CD26, preferably the CD26 extracellular domain; the amino acid sequence of the antibody or the fragment thereof comprises an amino acid sequence containing a monoclonal antibody or a fragment thereof or a conjugate of the fragment in any one of 6 complementary determining regions in one of SED ID NO: 2, SED ID NO: 3, SED ID NO: 4, SED ID NO: 6, SED ID NO: 7, and SED ID NO: 8, or an amino acid sequence obtained through amino acid replacement or modification. The obtained anti-CD26 single-chain antibody provided by the invention can highly specifically bind with the CD26 and is simultaneously capable of obviously inhibiting the proliferation, the invasion and the metastasis of tumor cells.
Owner:ZONHON BIOPHARMA INST
Preparation method of polypeptide for inhibiting gelatin enzyme A activity and its application
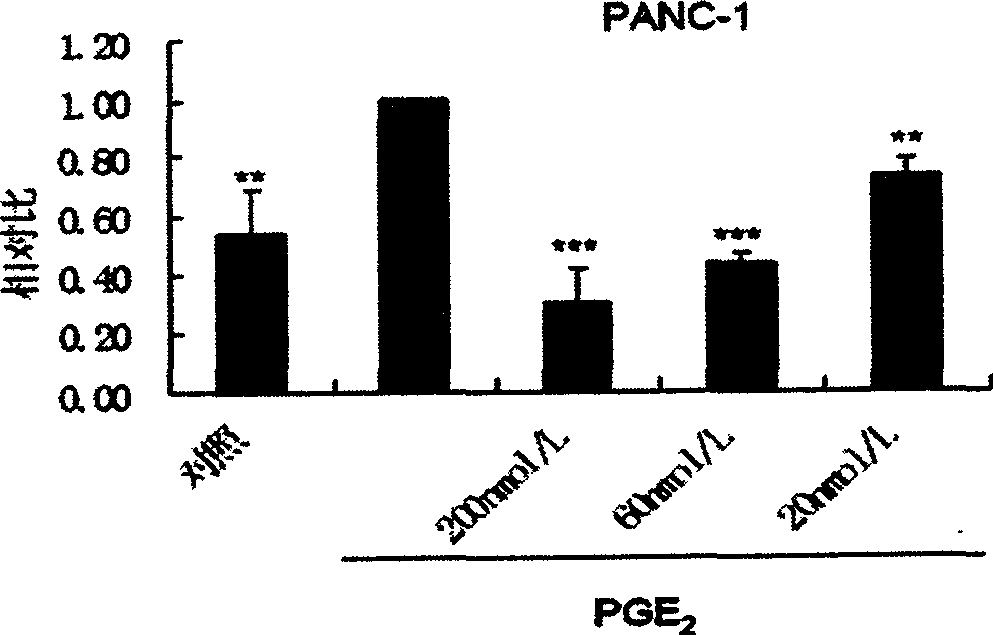
InactiveCN1869063AIncrease aggressivenessInhibition of invasion and metastasisPeptide/protein ingredientsMicrobiological testing/measurementPancreas CancersFluorescence
A polypeptide M204C4 for suppressing the activity of gelatinase A (MMP-2) is composed of the amino acid sequence shown by HNWTRWLLHPDRGGGS is disclosed. It can also suppress the invasion and transfer of external pancreas cancer cells PANC01 and CFPAC-1. Its preparing process includes such steps as affinity screening and reproduction of bacteriophage several times to obtain the bacteriophage with specific binding to MMP-2, coating, cloning and monoclonal screening with fluorescent screening reagent kit.
Owner:NANJING MEDICAL UNIV
Antineoplastic invasion transfer function of snake venom metalloprotease inhibitors BJ46a and uses thereof
InactiveCN101429525AInhibitory activityObvious anti-tumor invasion and metastasis effectPeptide/protein ingredientsGenetic material ingredientsAbnormal tissue growthIn vivo
The invention discloses a snake venom metal protease inhibitor BJ46a capable of resisting attack and transference of tumor and application thereof, and belongs to the field of medical organism. In the invention, according to the BJ46a gene sequence (AF294836) in GenBank, the BJ46a full gene is designed and synthesized. An expression vector cloned to baculovirus can generate the recombined BJ46a protein capable of inhibiting the activity of the substrate metal protease in Sf9 insect cells, and in vivo and in vitro experiments show that the BJ46a protein has the functions of resisting the attack and transference of melanoma cells B16. The inhibitor adopts the genetic transfection technique, and can establish B16 / pcDNA3.1HisC-BJ46a cell strains with stable transfection, and the in vivo and in vitro experiments prove that the BJ46a can inhibit the attack and transference of B16 cells at the gene level. The inhibitor is applied to preventing and treating the attack and transference of tumor, and has great application prospect.
Owner:FUJIAN MEDICAL UNIV
Polypeptide for inhibiting gelatin enzyme A activity and its preparation method and application
InactiveCN1869062AIncrease aggressivenessInhibition of invasion and metastasisSugar derivativesPeptide/protein ingredientsPancreas CancersFluorescence
A polypeptide M205C4 for suppressing the activity of gelatinase A (MMP-2) is composed of the amino acid sequence shown by HNWTRWLLHPDRGGGS is disclosed. It can also suppress the invasion and transfer of external pancreas cancer cells PANC01 and CFPAC-1. Its preparing process includes such steps as affinity screening and reproduction of bacteriophage several times to obtain the bacteriophage with specific binding to MMP-2, coating, cloning and monoclonal screening with fluorescent screening reagent kit.
Owner:NANJING SENMU BIOTECH CO LTD
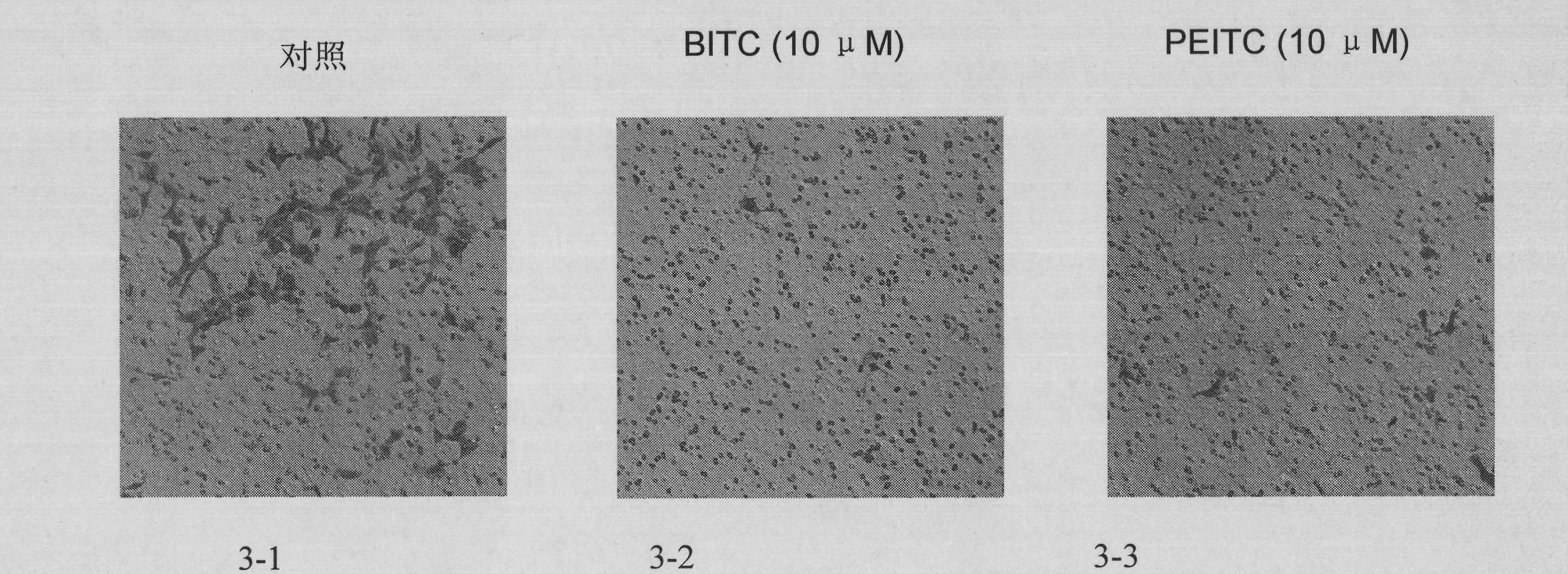
Usage of isothiocyanate in preparing medicine for preventing from tumor invasion and metastasis
ActiveCN101780066AGrowth inhibitionInhibition of invasion and metastasisEster active ingredientsAntineoplastic agentsAbnormal tissue growthLymphatic Spread
The invention discloses the usage of isothiocyanate in preparing a medicine for preventing from tumor invasion and metastasis. The usage proves that the isothiocyanate can effectively preventing from tumor cells invasion and metastasis by means of cytobiology and molecular biology. The isothiocyanate effectively restrains the growth and the invasion of the tumor cells, induces the tumor cells to be apoptosis, adjusts and controls the correlative genes of the tumor metastasis, induces the period of the tumor cells to be arrested, and damages the tumor cells caused by oxidation. Therefore, the isothiocyanate can be taken as an effective component to prepare medicines, foods, health care products and cosmetics for preventing from the tumor invasion and metastasis.
Owner:GENERAL HOSPITAL OF TIANJIN MEDICAL UNIV
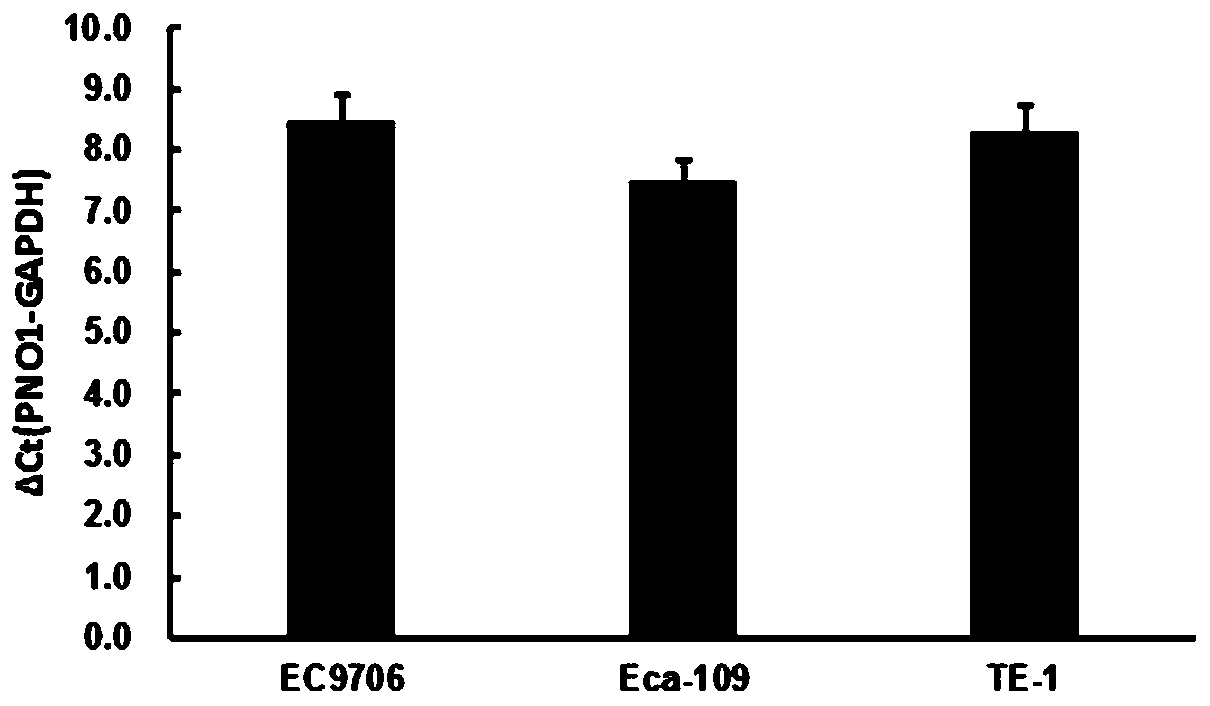
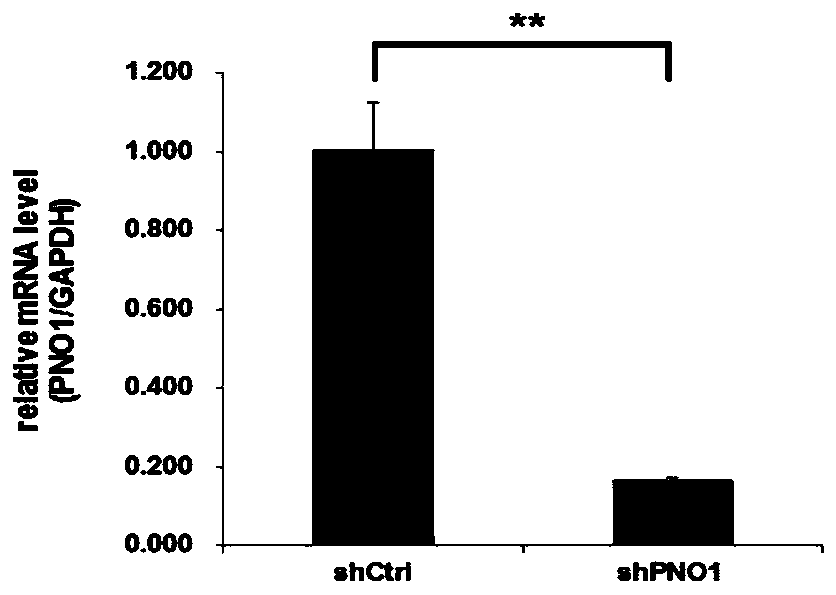
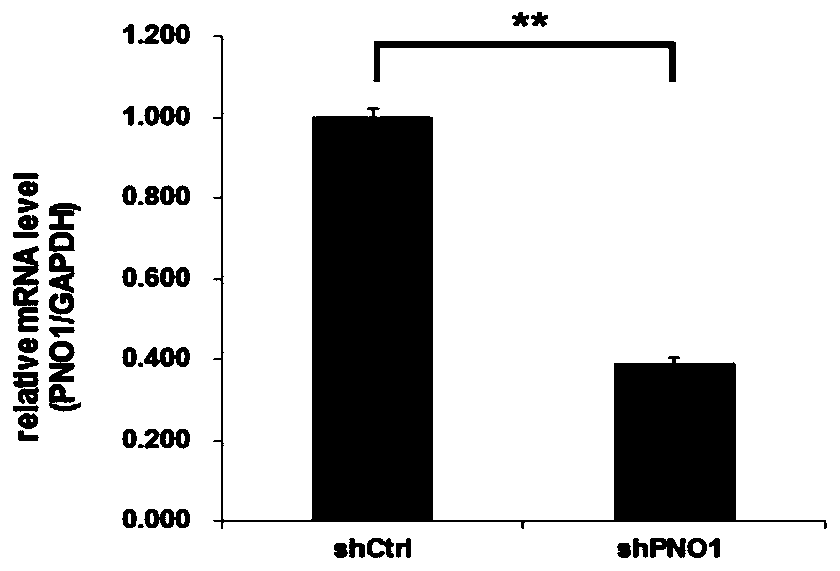
Application of PNO1 inhibitor to preparation of esophagus cancer treatment medicines
ActiveCN110656111AInhibition of proliferation rateInhibition of clonogenicityFermentationAntineoplastic agentsCell invasionPharmaceutical drug
The invention relates to an application of a PNO1 inhibitor to preparation of esophagus cancer treatment medicines, belongs to the field of biopharmaceutical research, and particularly relates to an application of a PNO1 inhibitor to preparation of esophagus cancer treatment medicines. The inventor firstly finds that the PNO1 can be used as an esophagus cancer treatment target point. The PNO1 inhibitor can restrain the proliferation rate of esophagus cancer cells, can restrain the cloning and forming capacity of the esophagus cancer cells, can promote esophagus cancer apoptosis, can restrain esophagus cancer cell migration, can restrain esophagus cancer cell transfer, and can restrain esophagus cancer cell invasion and transfer, so as to treat esophagus cancer, and a new direction is opened up for treatment of esophagus cancer.
Owner:THE FIRST AFFILIATED HOSPITAL OF BENGBU MEDICAL COLLEGE
Recombinant fusion resisting tomur attack and transfer
InactiveCN1451666AInhibition of invasion and metastasisGrowth inhibitionPeptide/protein ingredientsFermentationMutantBacteria
A recombinant fusion protein able to resist against tumor invasion and transfer is composed of the recombinant urokinase fragment and type-2 mutant as plasminogen activator inhibitor. The fusion protein gene cDNA is amplified by PCR method, and is cloned to expression vector to obtain fusion gene expression plasmid, which is used to transform host bacteria. The methanol or temp can induce the expression of said fusion protein. After purified, a high-purity (95%) expression product is obtained.
Owner:FUDAN UNIV
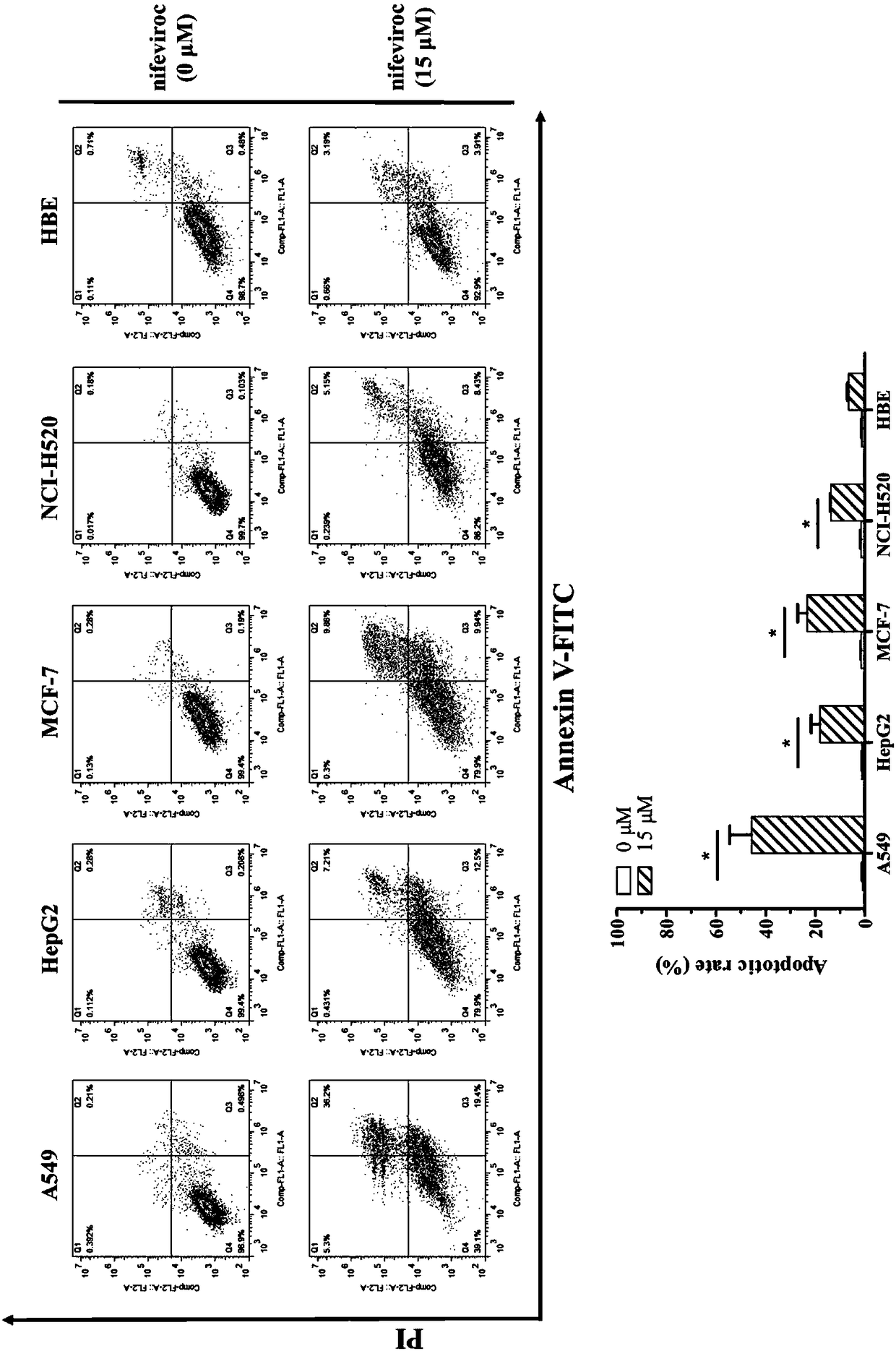
Application of Nifeviroc in preparing antitumor drug
InactiveCN108379268AHigh affinityHigh activityOrganic active ingredientsAntineoplastic agentsLymphatic SpreadSide effect
The invention provides application of Nifeviroc in preparing an antitumor drug, and provides an antitumor drug containing Nifeviroc. As is proved by cell experiments, after Nifeviroc acts on tumor cells, the cell cycle progress can be interfered, and the cell growth is inhibited, so that the cell cycle is stagnant at the G0-G1 stage; meanwhile, cell apoptosis can also be induced by increasing theactive oxygen content in the cells and lowering the mitochondrial membrane potential; meanwhile, Nifeviroc has an obvious effect of inhibiting invasion and metastasis of the tumor cells, by inhibitingadhesive power of the tumor cells to an extracellular matrix, the directed migration capability, the non-targeted migration capability and the in-vitro invasion capability of the cells are lowered, and the increase in size of the tumors in vivo can also be inhibited. As can be seen, Nifeviroc has a good effect of resisting the activity of the tumor, can be used for preparing the antitumor drug, does not have an obvious side effect, and has a good application prospect.
Owner:CHONGQING UNIV OF TECH
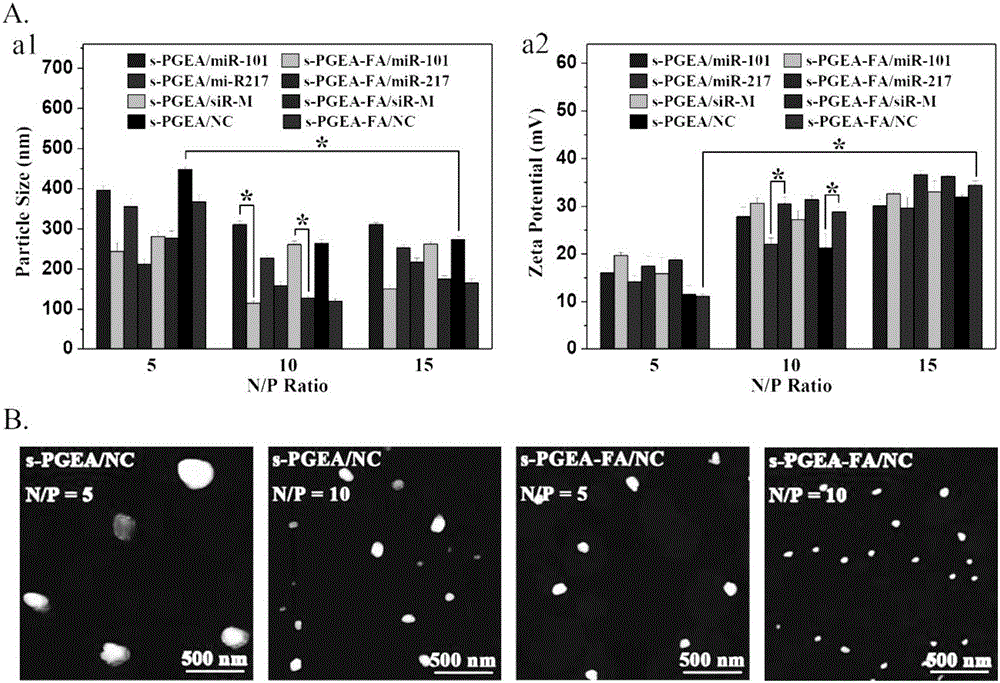
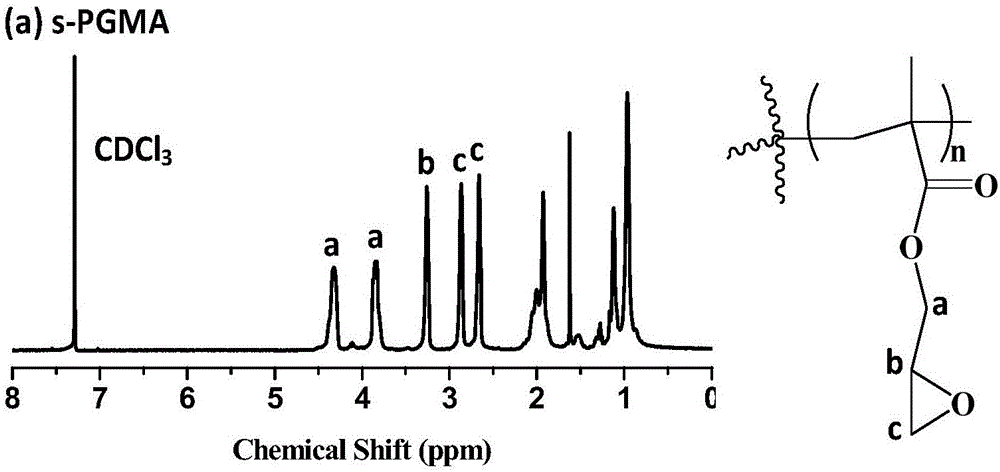
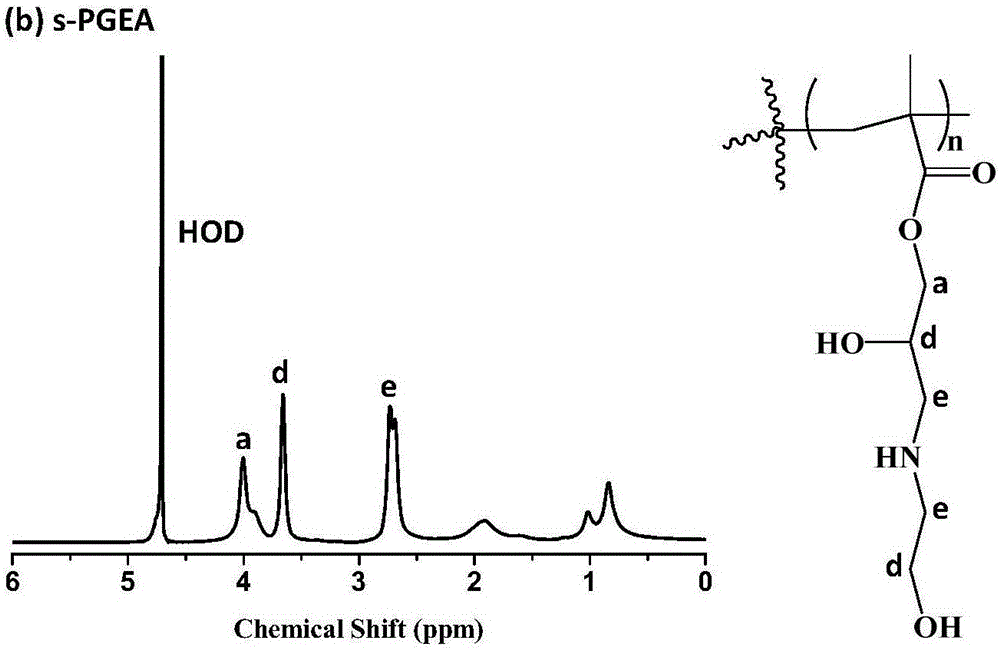
Hydroxyl-enriched nano gene vector as well as preparation method and application thereof
InactiveCN106755111AEfficient killingInhibition of invasion and metastasisOther foreign material introduction processesLymphatic SpreadPentaerythritol
The invention relates to a hydroxyl-enriched nano gene vector as well as a preparation method and application thereof. The vector adopts pentaerythritol as a core, has four arms and is of a star-shaped structure, and the arms have equal molecular weights. The invention discloses the nano gene vector based on a star-shaped polycation derivative (s-PGEA-FA) for a first time, lateral group of the nano gene vector comprises FA ligand and / or rich hydrophilic hydroxyl and secondary amine groups, and the vector is synthesized through atom transfer radical polymerization, ring-opening reaction and amidation reaction, is capable of mediating efficient delivery of miR-101 and miR-217 in different esophagus cancer cell systems, is capable of efficiently silencing the expression of lncRNA MALAT1 and furthermore killing esophagus cancer cells and inhibiting invasion and metastasis of the esophagus cancer cells, and has wide application prospects in treatment on esophagus cancer.
Owner:SHANDONG RES INST OF TUMOUR PREVENTION TREATMENT
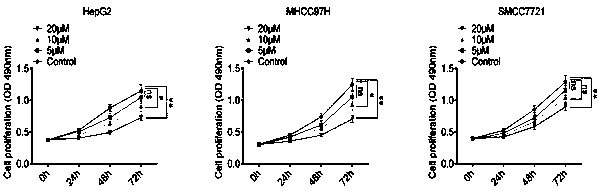
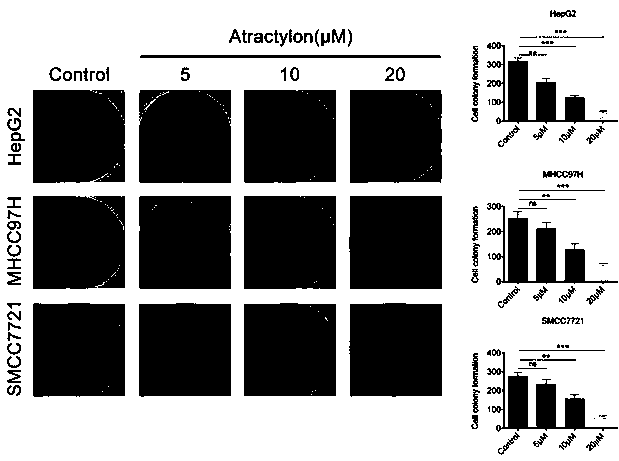
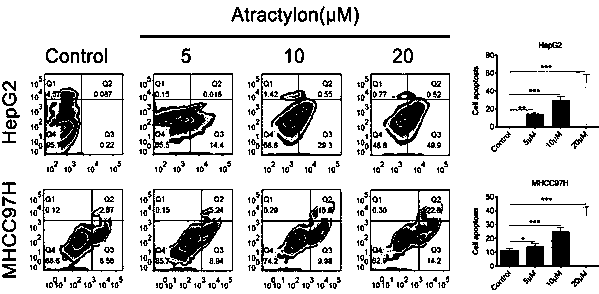
Application of atractylon to preparation of anti-liver cancer molecular target medicine
InactiveCN109157533AInhibition of invasion and metastasisGrowth inhibitionOrganic active ingredientsAntineoplastic agentsLymphatic SpreadStructural formula
The invention discloses application of atractylon shown as a structural formula (I) to preparation of an anti-liver cancer molecular target medicine. Seen from results of cell experiments and animal experiments provided by the invention, the atractylon has the property and the effect of inhibiting invasion and metastasis of liver cancer cells, which are found by the invention for the first time; in addition, the animal experiments further prove that the atractylon has the function of inhibiting growth of liver tumors in a nude mouse, has a significant anti-tumor effect on the nude mouse, and reaches an unexpected technical effect in the field. These results indicate that the atractylon can be applied to preparation of the anti-liver cancer molecular target medicine and has good applicationprospect and good application value.
Owner:上海市浦东新区传染病医院
Cationic liposome compound of cancer suppressor gene LKB1 eukaryotic expression plasmid as well as preparation method and anti-tumor effect thereof
InactiveCN103045634AReduce the numberGrowth inhibitionGenetic material ingredientsTransferasesLipofectamineInvasion metastasis
The invention belongs to the field of gene treatment, provides a new gene therapy product, and specifically relates to a cationic liposome compound of cancer suppressor gene LKB1 eukaryotic expression plasmid as well as a preparation method and an anti-tumor effect thereof. The gene expression carrier contains genes encoding human LKB1 protein and can express the human LKB1 protein in eukaryotic cells. Based on experiments, the cationic liposome compound of cancer suppressor gene LKB1 eukaryotic expression plasmid, disclosed by the invention, has excellent functions of resisting tumor growth and invasion and metastasis so as to provide a new selection for the tumor treatment.
Owner:SICHUAN UNIV
Polypeptide for inhibiting gelatin enzyme A activity and its preparation method and application
InactiveCN100383161CIncrease aggressivenessInhibition of invasion and metastasisSugar derivativesPeptide/protein ingredientsPancreas CancersFluorescence
A polypeptide M205C4 for suppressing the activity of gelatinase A (MMP-2) is composed of the amino acid sequence shown by HNWTRWLLHPDRGGGS is disclosed. It can also suppress the invasion and transfer of external pancreas cancer cells PANC01 and CFPAC-1. Its preparing process includes such steps as affinity screening and reproduction of bacteriophage several times to obtain the bacteriophage with specific binding to MMP-2, coating, cloning and monoclonal screening with fluorescent screening reagent kit.
Owner:NANJING SENMU BIOTECH CO LTD
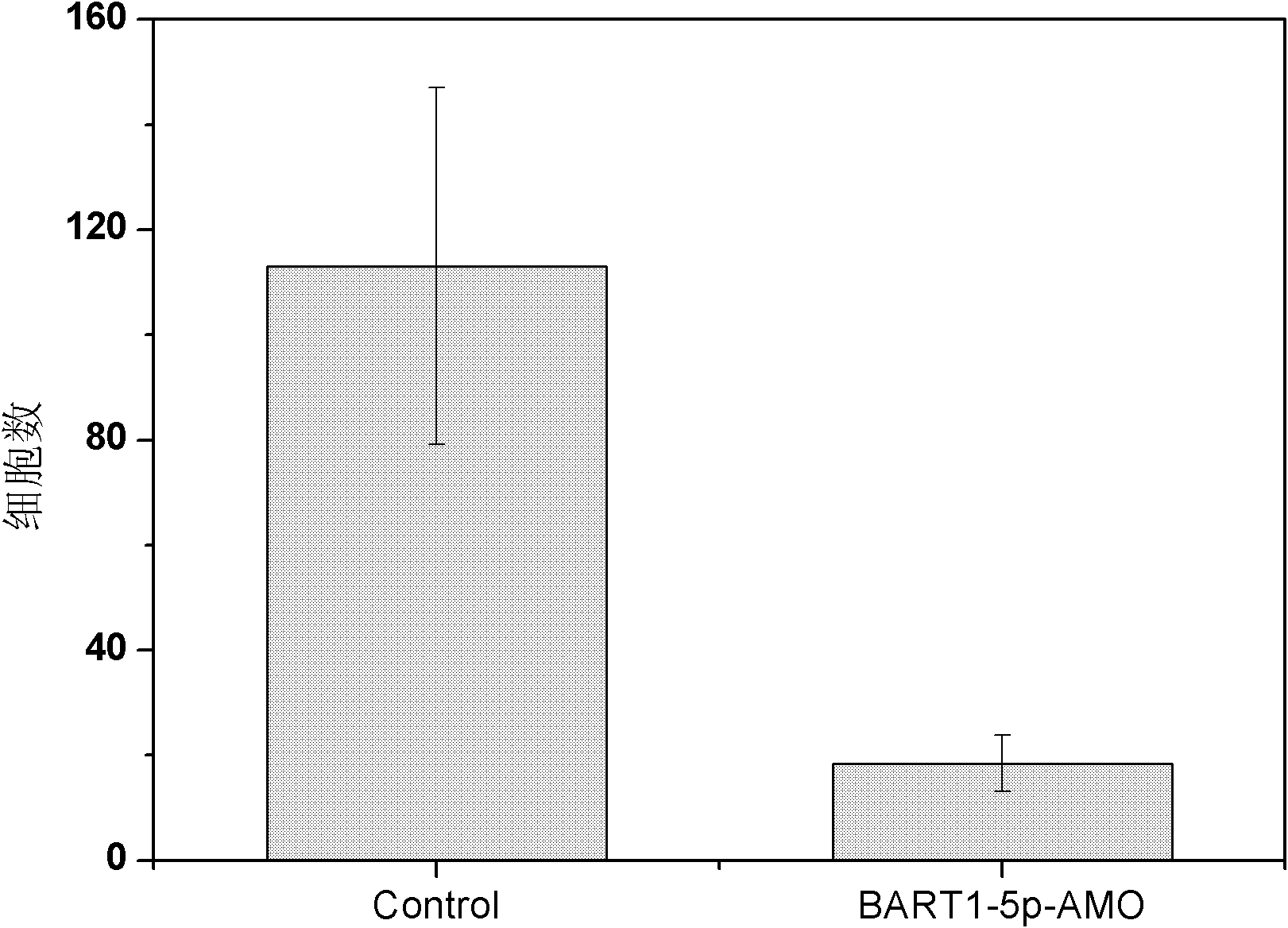
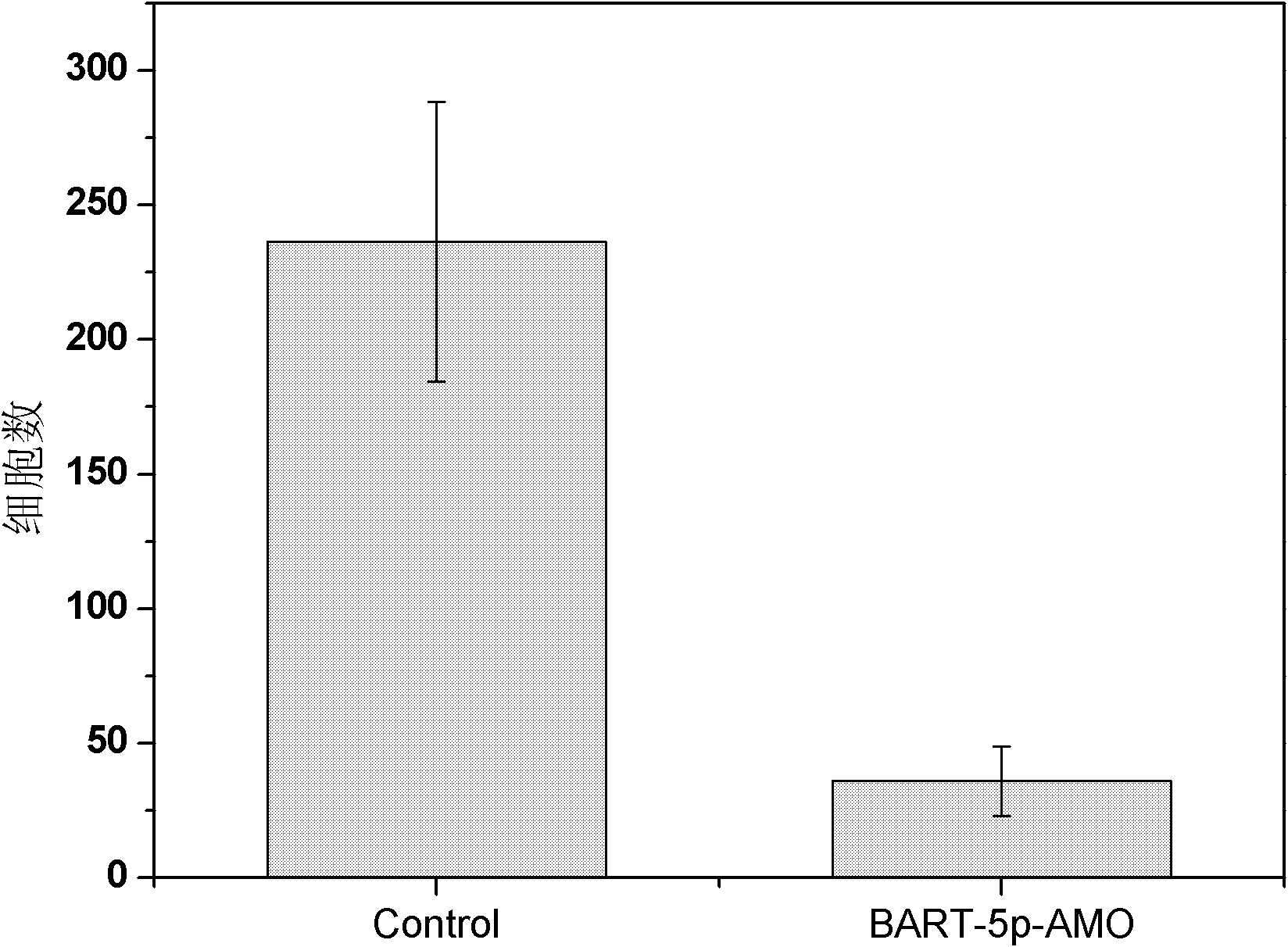
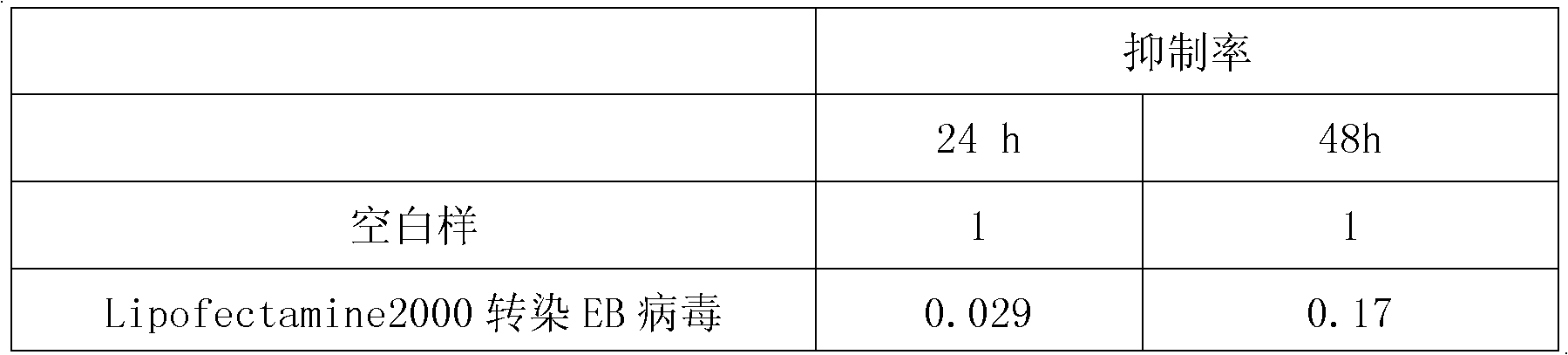
Use of epstein-barr virus miR-BART1-5p antisense oligonucleotide in preparing drugs for treating nasopharyngeal carcinoma
InactiveCN102218148AProlong the action timeNon-immunogenicGenetic material ingredientsInorganic non-active ingredientsNucleotideNasopharyngeal carcinoma
Owner:SOUTHERN MEDICAL UNIVERSITY
Multifunctional bionic HA particles loaded curcumin prodrug micro-nano composite material, preparation method and application thereof
ActiveCN112057671ABoth tumor suppressorWith bone-promoting and repairing propertiesTissue regenerationPhosphorus compoundsMicro nanoFreeze-drying
The invention discloses a multifunctional bionic HA particles loaded curcumin prodrug micro-nano composite material, a preparation method and an application thereof. The method comprises the followingsteps, preparing hydroxyapatite micro-nano particles with a bionic structure; preparing a curcumin nano prodrug; adding hydroxyapatite powder and the curcumin nano prodrug into water, uniformly mixing the mixture, and carrying out incubating, adsorbing, centrifuging, freeze-drying and sterilizing to obtain the composite micro-nano particles. The prepared composite micro-nano particles of the present invention have high drug loading capacity, a selective targeting function, good biocompatibility and no obvious toxic or side effect on living organisms. In high drug loading capacity, the composite particles can effectively inhibit growth of bone tumors. In minor drug loading capacity, the micro-nano multistage structure of the composite particles can efficiently promote regeneration and repair of bone tissues.
Owner:SOUTH CHINA UNIV OF TECH
Application of skeleton protein source polypeptide in preparing medicines for restraining invasion and transfer of colorectal cancer cells
InactiveCN101850107AGood inhibitory effectInhibition of invasion and metastasisPeptide/protein ingredientsDigestive systemMedicineInhibitory effect
The invention discloses an application of skeleton protein source polypeptide in preparing medicines for restraining the invasion and the transfer of colorectal cancer cells. The V+Repeat fused polypeptide has very obvious restraining action on the invasion and the transfer of the colorectal cancer.
Owner:AFFILIATED HOSPITAL OF NANTONG UNIV
Application of HIC1 (hypermethylated in cancer 1) to diagnosis, treatment, prognosis and recurrence prediction of tumors
InactiveCN107267597AReduce tumorigenesisImprove survival rateMicrobiological testing/measurementAntineoplastic agentsLymphatic SpreadProstate cancer
The invention discloses an application of HIC1 (hypermethylated in cancer 1) to diagnosis, treatment, prognosis and recurrence prediction of tumors. The invention discovers that the copy number and the expression level of HIC1 in various tumors are closely related with invasion, metastasis, recurrence and prognosis of the tumors. Research finds that decreased expression of HIC1 caused by hypermethylation of HIC1 can promote invasion and metastasis of the tumors, and the expression decrease degree of HIC1 is in negative correlation with prognosis of patients. By restoring expression of HIC1, invasion and metastasis abilities of breast cancer, prostate cancer and lung cancer can be effectively weakened, and the survival rate of the patients is increased, so that HIC1 can serve as a marker for diagnosis, treatment, prognosis and recurrence prediction of the tumors, and a kit can be prepared from HIC1. Drugs capable of promoting HIC1 expression have great clinical application value in preparation of tumor treatment pharmaceutical composition, and novel drugs and methods are provided for effective treatment of the tumors.
Owner:王建华
Polynucleotide for tumor treatment and application of polynucleotide
ActiveCN109486816AInhibition of invasion and metastasisInhibit the metastasis of gastric cancerOrganic active ingredientsAntineoplastic agentsChemical synthesisFhit gene
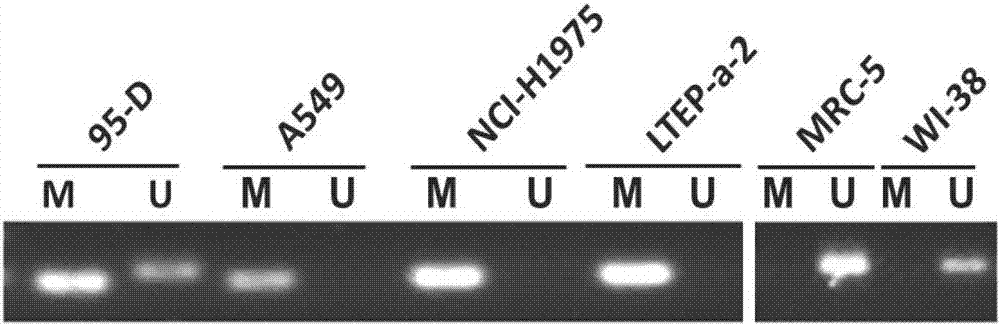
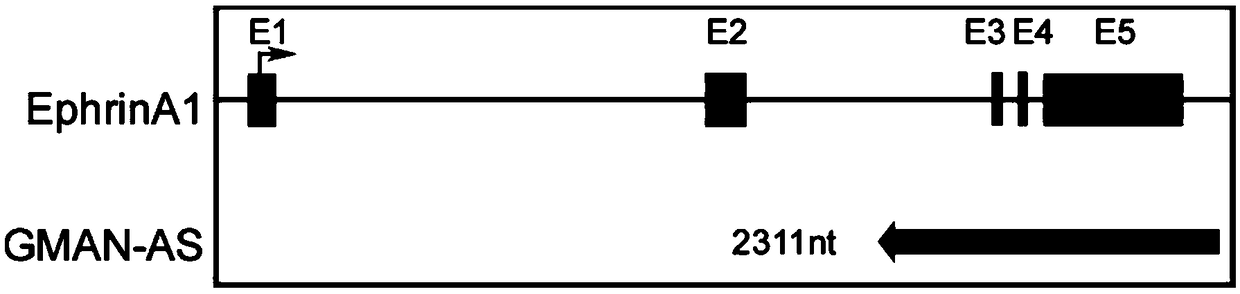
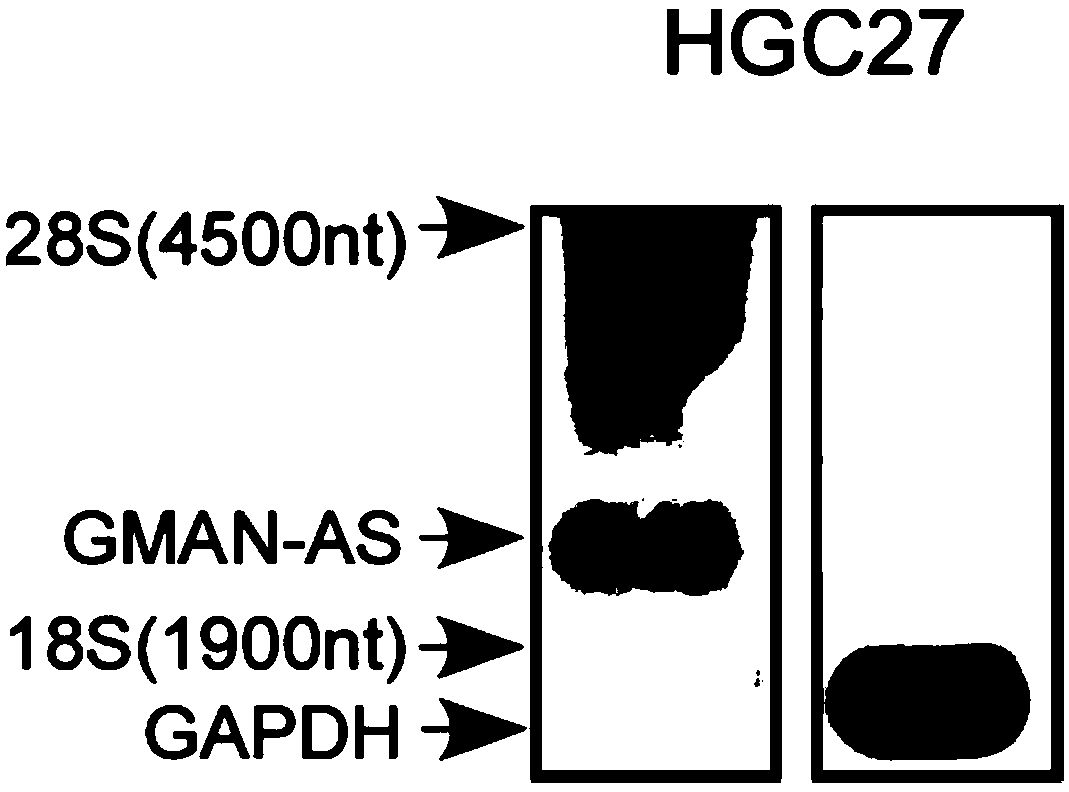
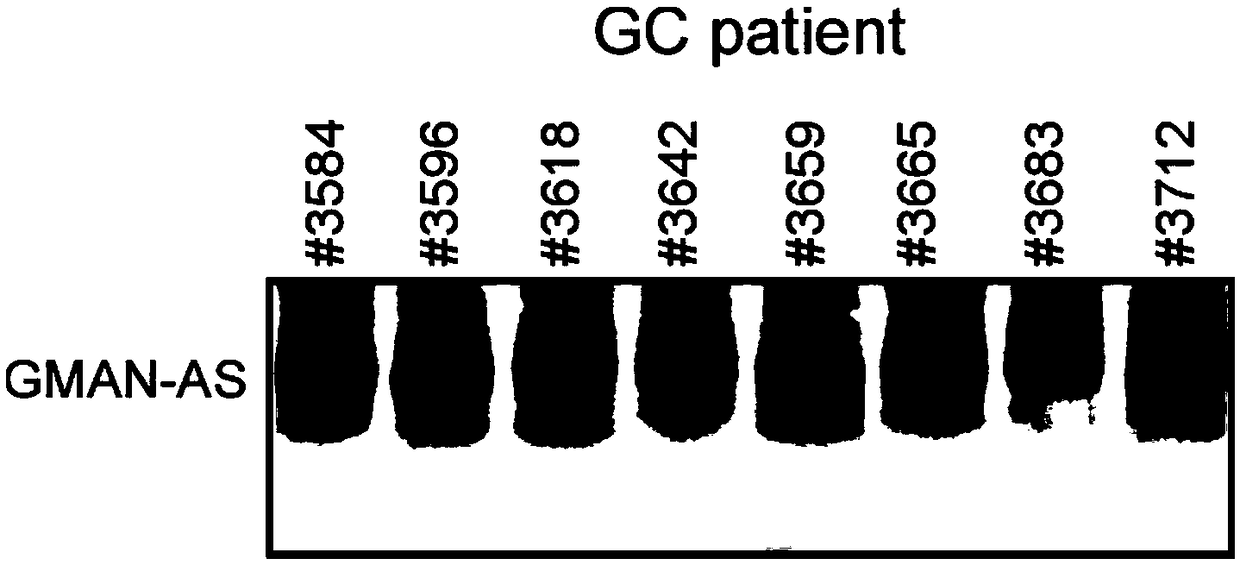
The invention discloses a polynucleotide for tumor treatment. The polynucleotide comprises an MR fragment shown in SEQ ID NO.1 or further comprises an AMFR fragment shown in SEQ ID NO.2, wherein, theMR segment is a 565 bp nucleotide sequence, and the AMFR segment is a 293 bp nucleotide sequence. Both chemical synthesis and targeted gene editing technologies have the characteristics of simple operation and strong feasibility. The individual MR fragment, or AMFR fragment, and GMAN-AS containing the MR fragment and the AMFR fragment can significantly inhibit the invasion of gastric cancer cells,so that the polynucleotide has important potential for treating gastric cancer metastasis.
Owner:ZHEJIANG UNIV
Biscuits rich in zinc and selenium elements
InactiveCN105875739AGood anticancer effectWith hypolipidemicDough treatmentBakery productsBiotechnologyDigestion
The invention relates to biscuits rich in zinc and selenium elements. The biscuits comprise the following components in parts by weight: 42-50 parts of selenium-enriched red rice flour, 15-17 parts of selenium-enriched Chinese yam powder, 5-6 parts of longan, 12-15 parts of milk powder, 4-6 parts of syrup, 8-10 parts of peanut oil, 1-1.2 parts of vitamins and mineral substances, 2-3 parts of a swelling agent and 1-1.8 parts of table salt. Compared with the prior art, the biscuits disclosed by the invention are good in mouth feel, rich in various elements, beneficial to digestion, rich in nutrition and simple to make, and cannot enable people to feel dried when being eaten.
Owner:包宗辉
Gold nano composite material of high target tumor, and preparation method and application of gold nano composite material
InactiveCN106924218AImprove targetingInhibition of invasion and metastasisOrganic active ingredientsInorganic non-active ingredientsDouble strandedDoxorubicin
The invention discloses a gold nano composite material of a high target tumor, and a preparation method and application of the gold nano composite material. The gold nano composite material is prepared from doxorubicin, AMD3100, double-chain DNA (deoxyribonucleic acid), PEG (polyethylene glycol) and gold nano particles; the doxorubicin is loaded on the double-chain DNA, and the AMD3100 is modified on the PEG; and the double-chain DNA and the PEG are connected onto the gold nano particles by virtue of sulfydryl. According to the gold nano composite material of the high target tumor, the anticancer drug doxorubicin is used for achieving a chemotherapy effect on tumor cells, and a small molecular inhibitor AMD3100 can be combined with a CXCR4 receptor highly expressed on the surface of the tumor cells in a targeting way. Since the AMD3100 can be combined with and can lower the expression of the CXCR4 and can also inhibit the invasion transfer of the tumor, the combination of the AMD3100 and the CXCR4 can effectively improve an effect for killing the liver cancer, can reduce the consumption of the doxorubicin, and can reduce the toxicity and adverse effect for the normal cells by specifically targeting a liver cancer tissue.
Owner:THE SECOND AFFILIATED HOSPITAL ARMY MEDICAL UNIV
Long-acting acne removal skincare composition and application thereof
InactiveCN108420765AInduce apoptosisInhibition of invasion and metastasisCosmetic preparationsToilet preparationsAbnormal hormone secretionMedicine
The invention discloses a long-acting acne removal skincare composition. The long-acting acne removal skincare composition comprises, by weight, 0.1-10 parts of ononis spinosa, 0.1-10 parts of viscumcoloratum, 0.1-10 parts of carica papaya, 0.1-10 parts of drynaria fortunei and 0.1-10 parts of trigonella foenum-graecum. The long-acting acne removal skincare composition has the advantages that various medicines are used with one another, synergistic effects can be realized, abnormal hormone secretion can be regulated, the internal ecological balance of hair follicles can be regulated, accordingly, acne can be removed in a long-acting manner, and the problem of recurrent attack of acne can be solved by the aid of the acne removal skincare composition.
Owner:FOSHAN YUNSHANG COSMETICS CO LTD
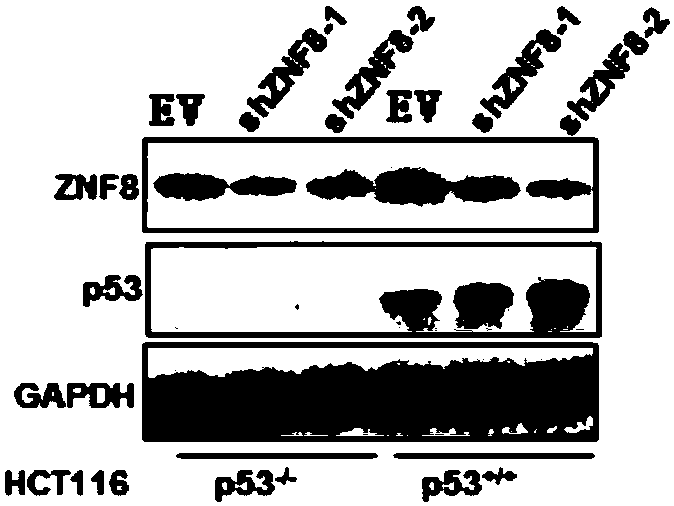
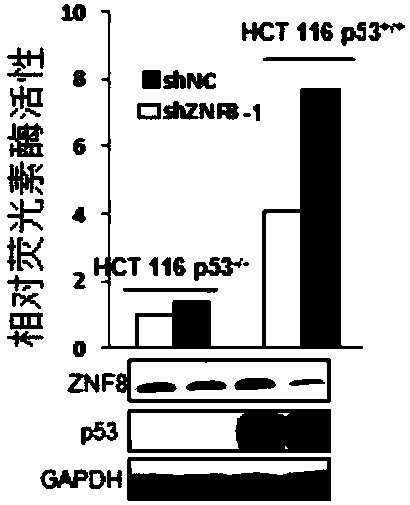
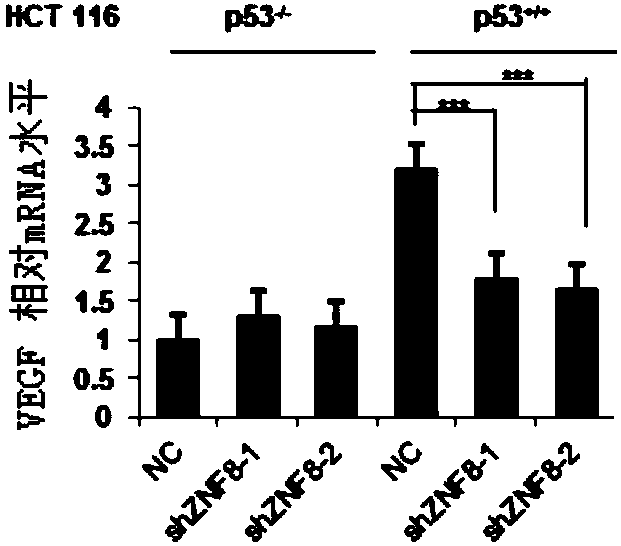
Application of substance for inhibiting expression of ZNF8 protein in preparing products for preventing and treating cancer
PendingCN109806401AIncrease transcriptional activityPrevent proliferationOrganic active ingredientsGenetic material ingredientsP53 proteinWilms' tumor
The invention discloses application of a substance for inhibiting the expression of ZNF8 protein in preparing products for preventing and treating cancer. An experiment shows that the ZNF8 protein canbe bound with p53 protein; by inhibiting the expression of the ZNF8 protein, the transcription activity of the p53 protein can be increased, the expressions of a VEGF gene and an ANGPLT4 gene are reduced, and proliferation, metastasis, migration, invasion and metastasis of tumor cells are inhibited; the formation of blood vessels is inhibited; the growth,the metastasis and lung metastasis of thetumor are inhibited; the survival rate of tumor-carrying animals is improved. Therefore, inhibition of the expression of the ZNF8 protein has important application value in inhibiting the growth and invasive metastasis of the tumor. Cancer can be prevented and / treated by inhibiting the expression of the ZNF8 protein.
Owner:BEIJING PROTEOME RES CENT
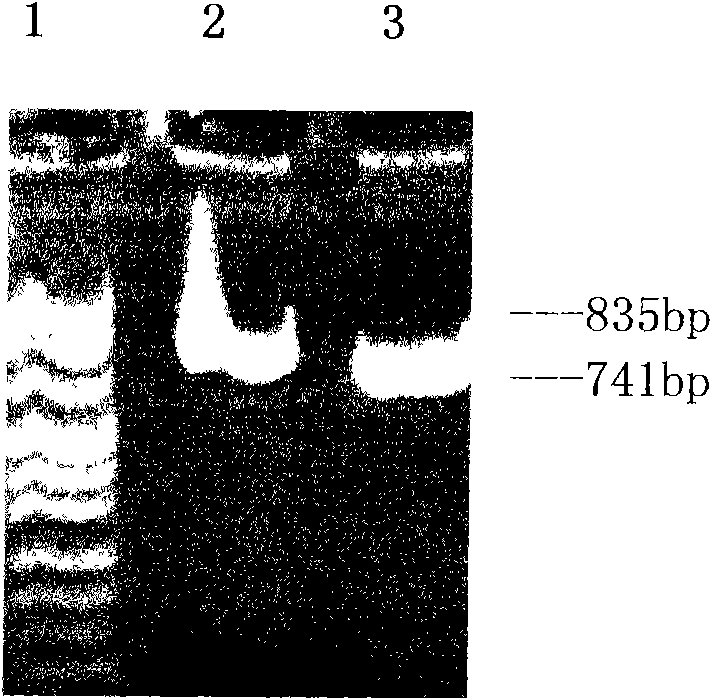
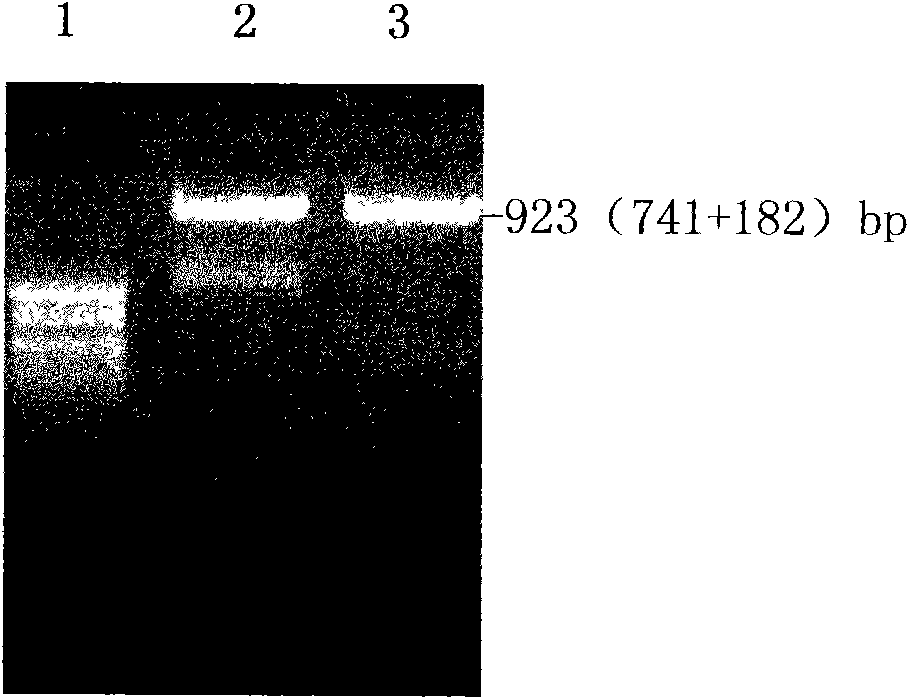
Application of recombinant FN heparin binding domain polypeptide on preparing medicament resisting invasion and metastasis of malignant tumor
ActiveCN101596307AHigh activityPromote generationPeptide/protein ingredientsAntineoplastic agentsYeastEnzyme digestion
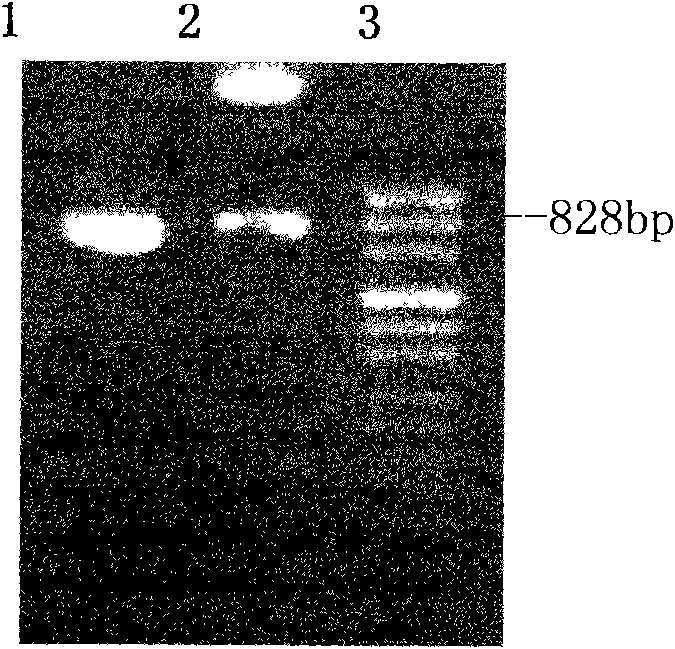
The invention discloses an application of recombinant FN heparin binding domain polypeptide on preparing medicaments resisting the invasion and the metastasis of malignant tumors. The recombinant FN heparin binding domain polypeptide comprises five I type homologous structures at the end N in FN molecules and contains 237 amino acids in Ser46-Gly 282. DNA sequences for encoding the polypeptide range from 403bp to 1113bp and have the length of 711bp, a PCR amplified fragment has the length of 741bp, and the length of the fragment after double enzyme digestion is 729bp. The molecular weight of the polypeptide expressed by yeast is 28.52kDa, and the polypeptide comprises three III type homologous structures in the FN molecules, i.e. III-12, III-13 and III-14 and contains 272 amino acids in Tyr1720-Tyr1991. DNA sequences for encoding the polypeptide range from 5428bp to 6244bp and have the length of 816bp, a PCR amplified fragment has the length of 835bp, and the length of the fragment after double enzyme digestion is 828bp. The molecular weight of the polypeptide expressed by the yeast is 36.09kDa. The experiments prove that the recombinant FN heparin binding domain polypeptide can be applied to preparing medicaments resisting the invasion and the metastasis of malignant tumors.
Owner:FUJIAN MEDICAL UNIV UNION HOSPITAL
Small peptide capable of inhibiting metastasis of liver cancer and its preparation method and application
ActiveCN112552390BPromote degradationImprove migration abilityPeptide/protein ingredientsAntineoplastic agentsCancer cellSmall peptide
The invention discloses a small peptide with the function of inhibiting liver cancer metastasis and its preparation method and application, wherein the small peptide has the amino acid sequence shown in SEQ ID NO.1 to 4 or any one of SEQ ID NO.1 to 4 The shown amino acid sequence is substituted, deleted and / or added with one or more amino acids and / or terminal modified and has the function of inhibiting liver cancer metastasis. The invention also provides a preparation method of the small peptide, and obtains the amino acid site that mediates its rapid degradation. The small peptide in the present invention is found to have the function of inhibiting the invasion and metastasis of liver cancer cells, has a strong ability to inhibit the migration of liver cancer cells, and has the application potential of developing new anti-metastasis drugs or other preparations of liver cancer cells.
Owner:SHENZHEN HOSPITAL OF SOUTHERN MEDICAL UNIV
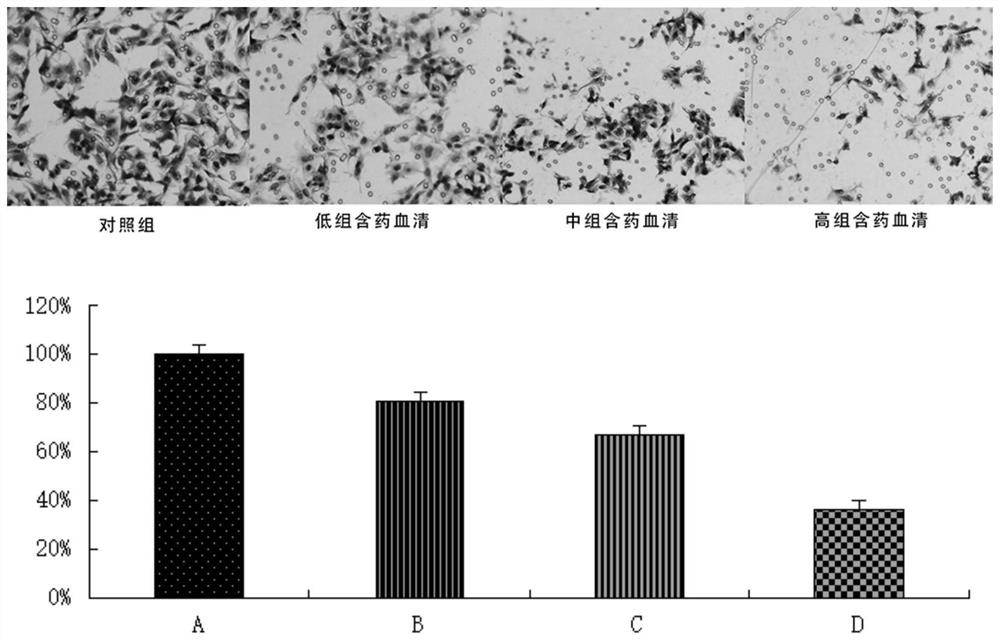
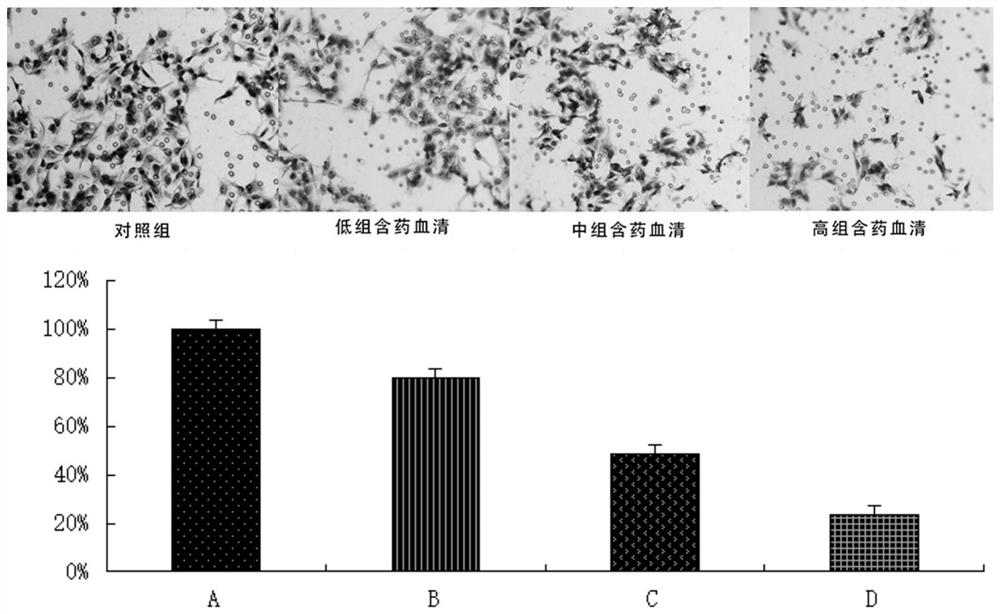
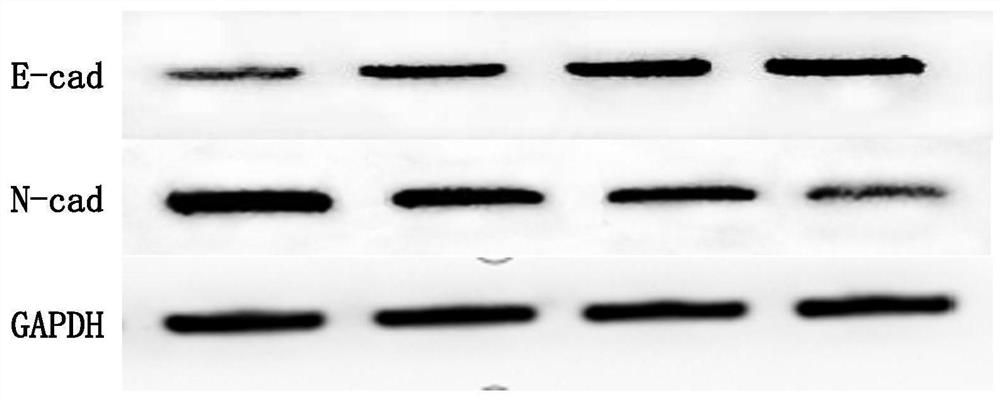
Application of pueraria flower spleen-strengthening-relieving formula drug-containing serum in cancer cells
PendingCN112263592AInhibition of invasion and metastasisInhibits the epithelial-mesenchymal transition processMammal material medical ingredientsFungi medical ingredientsDrugHepatoma cell
The invention discloses application of pueraria flower spleen-strengthening-relieving formula drug-containing serum in cancer cells. By preparing the pueraria flower spleen-strengthening-relieving formula drug-containing serum, observing the influence of the drug-containing serum with different concentrations on the invasion, transfer and epithelial-mesenchymal transition of HepG2 hepatoma carcinoma cells and observing the influence of the drug-containing serum with different concentrations on the proliferation and apoptosis of the HepG2 hepatoma carcinoma cells, the pueraria flower spleen-strengthening-relieving formula drug-containing serum is proved to have the effects of inhibiting the proliferation of the HepG2 hepatoma carcinoma cells, promoting the apoptosis of the HepG2 hepatoma carcinoma cells and the like, and theoretical and data support is provided for the clinical anti-hepatoma metastasis application of the formula.
Owner:贵州中医药大学
Usage of isothiocyanate in preparing medicine for preventing from tumor invasion and metastasis
ActiveCN101780066BGrowth inhibitionInhibition of invasion and metastasisEster active ingredientsAntineoplastic agentsAbnormal tissue growthLymphatic Spread
The invention discloses the usage of isothiocyanate in preparing a medicine for preventing from tumor invasion and metastasis. The usage proves that the isothiocyanate can effectively preventing from tumor cells invasion and metastasis by means of cytobiology and molecular biology. The isothiocyanate effectively restrains the growth and the invasion of the tumor cells, induces the tumor cells to be apoptosis, adjusts and controls the correlative genes of the tumor metastasis, induces the period of the tumor cells to be arrested, and damages the tumor cells caused by oxidation. Therefore, the isothiocyanate can be taken as an effective component to prepare medicines, foods, health care products and cosmetics for preventing from the tumor invasion and metastasis.
Owner:GENERAL HOSPITAL OF TIANJIN MEDICAL UNIV
Agastache rugosus plant fermentation solution and preparation method thereof
InactiveCN106511875AGrowth inhibitionWith hypolipidemicDispersion deliveryDigestive systemSide effectSeparation technology
The invention discloses an agastache rugosus plant fermentation solution and a preparation method thereof and relates to the technical field of fermentation solutions. The agastache rugosus plant fermentation solution comprises the following components: 5-7 parts of dry agastache rugosus, 5-7 parts of dry fructus amomi rotundus, 5-7 parts of dry fructus crataegi, 0.5-1 part of honey, 0.5-1 part of oligosaccharide, 0.5-1 part of glucose powder, 0.02-0.04 part of dry yeast and 50 parts of water. The preparation method of the agastache rugosus plant fermentation solution comprises the following steps: crushing the dry agastache rugosus, the dry fructus amomi rotundus and the dry fructus crataegi, then blending crushed dry agastache rugosus, dry fructus amomi rotundus and dry fructus crataegi with the honey, the oligosaccharide, the glucose powder and the dry yeast according to a ratio by using the water, adding the materials into a tank, sealing and fermenting until vinegar flavor is emitted, then processing, extracting and purifying a fermentation solution by using a supercritical fluid extraction separation technology to obtain the agastache rugosus plant fermentation solution. The fermentation solution is formed by fermenting pure traditional Chinese medicines, has vinegar flavor, is delicious with slight sweetness and sour, is good in taste, is popular to children, and is free of side effects after being taken. Clinical test shows that the fermentation solution is used for treating anorexia; the curative effect is obvious; the total effective rate is 95%.
Owner:LIUZHOU JINCHEN TECH
Tumour-dissolving adenovirus mutant possessing multiple specific anti-tumour mechanism
InactiveCN100582232CGood curative effectPlay a therapeutic roleGenetic engineeringFermentationHuman tumorReverse transcriptase
This invention involved oncolytic adenovirus mutant with the multiple antitumoral Mec. It belongs to the BME field. The adenovirus mutant Elb-55kDa and Elb-19kDa has missing gene, inserts chimeric promoter composed by the human telomerase reverse transcriptase core sequence and human tumor epidermal growth factor receptor enhancer before the replication required gene Ela codons mEla289R and mEla243R. So the virus can specific proliferate in cancer cell. Oncolytic viruses and mEla protein can exert thire influence. It can also increase the sensitivity of cancer cell to chemoradiation and have no influence to normal cell. It inserts the human h-endostatin gene expression cassette in adenovirus mutant gene group; along the replication of virus in cancer cell to amplificate h-endostatin gene and effective expression in tumor so to restrain neovascularization of tumor so to realize the result of restrain the increase and transformation of cancer cell and apoptotic cell. This new oncolytic adenovirus mutant has good clinical prospect in gene curing so to used in curing many kinds of human tumors.
Owner:JIANGSU SHUNTANG BIOENG
Small peptide with function of inhibiting liver cancer metastasis and preparation method and application of small peptide
ActiveCN112552390APromote degradationInhibition of invasion and metastasisPeptide/protein ingredientsAntineoplastic agentsLiver cancerSmall peptide
The invention discloses a small peptide with a function of inhibiting liver cancer metastasis as well as a preparation method and application of the small peptide. The small peptide has amino acid sequences shown as SEQ ID NO.1-4 or a sequence which is obtained by substituting, deleting and / or adding one or more amino acids to any one of the amino acid sequences shown as SEQ ID NO.1-4 and / or has afunction of inhibiting liver cancer metastasis after terminal modification. The invention further provides the preparation method of the small peptide, and an amino acid site mediating rapid degradation of the small peptide is obtained. The small peptide is found to have the function of inhibiting invasion and metastasis of liver cancer cells, is strong in capacity of inhibiting migration of theliver cancer cells, and has the application potential of developing novel liver cancer cell metastasis resisting drugs or other preparations.
Owner:SHENZHEN HOSPITAL OF SOUTHERN MEDICAL UNIV
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com