Method for removing endotoxin in human urokinase raw material refining process
A technology for urokinase and endotoxin, which is applied in the directions of biochemical equipment and methods, ion exchange column/bed methods, enzymes, etc., can solve the problems of reducing production costs, complex methods, increasing costs, etc. Simple, material-saving effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
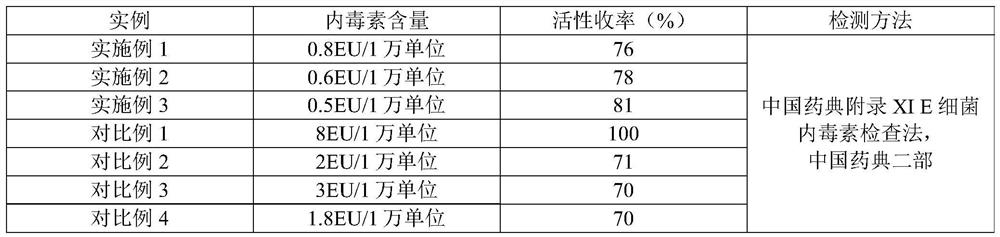
Embodiment 1
[0031] A method for removing endotoxin during the refining process of human urokinase raw materials, comprising the following steps:
[0032] (1) Sample pretreatment: adjust the pH of the raw material intermediate solution of human urokinase to 6.0 and the conductance to 1ms / cm;
[0033] (2) Chromatographic column preparation and packing: add water to the TOYOPEARL ion exchange resin superQ-650M of Tosoh Co., Ltd., Japan, to obtain a suspension, pack the column, and obtain the chromatographic column; wherein, the column height of the packing is 10cm , the diameter of the chromatographic column is 1.6cm; wherein, the specific steps of packing the column are: replace the preservation solution in the chromatographic filler with water for injection, a total of three times, adjust the chromatographic filler after the last replacement: the ratio of water is 1: 0.4; Start the chromatography system, drain the air in the chromatography system and the column head screen with water for i...
Embodiment 2
[0038] A method for removing endotoxin during the refining process of human urokinase raw materials, comprising the following steps:
[0039] (1) Sample pretreatment: adjust the pH of the raw material intermediate solution of human urokinase to 9.0 and the conductance to 10ms / cm;
[0040] (2) Chromatographic column preparation and column packing: add water to the SOURCE 30Q high-speed low back pressure ion exchange medium of U.S. General Electric Company, obtain suspension, pack column, obtain chromatographic column; Wherein, the column height of packing is 30cm, and the diameter of the chromatographic column is 30cm; wherein, the specific steps of packing the column are: replace the preservation solution in the chromatographic filler with water for injection, a total of three times, adjust the chromatographic filler after the last replacement: the ratio of water is 1: 1. Start the chromatography system, drain the air in the chromatography system and the column head screen wit...
Embodiment 3
[0045] A method for removing endotoxin during the refining process of human urokinase raw materials, comprising the following steps:
[0046] (1) Sample pretreatment: adjust the pH of the raw material intermediate solution of human urokinase to 8.0 and the conductance to 5ms / cm;
[0047] (2) Chromatographic column preparation and column packing: add water to the Chromalite MQ / F weak base anion resin of British Brilliant Resin Co., Ltd. to obtain a suspension, pack the column, and obtain the chromatographic column; wherein, the column height of the packed column is 20cm, and the diameter of the chromatography column is 20cm; wherein, the specific steps of packing the column are: replace the preservation solution in the chromatography filler with water for injection, a total of three times, adjust the chromatography filler after the last replacement: the ratio of water is 1 :0.8; start the chromatography system, drain the air in the chromatography system and the column head scre...
PUM
| Property | Measurement | Unit |
|---|---|---|
| diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com