Recombinant human prourokinase preparation for injection as well as preparation method and application thereof
A technology for prourokinase and injection, which is applied in the field of biomedicine, can solve the problems of taking up the rescue time of medical staff, low work efficiency, large supply of raw materials, etc., and achieves the effects of saving rescue time, improving work efficiency, and improving compliance.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0046] The present embodiment provides a liquid preparation of recombinant human urokinase for injection and a freeze-dried preparation, and the preparation method is as follows:
[0047] (1) Weigh recombinant human prourokinase, trehalose, polysorbate 80, citric acid / sodium citrate and mix and dissolve with water for injection, so that recombinant human prourokinase, trehalose, polysorbate 80, citrate The concentrations of acid / sodium citrate were respectively 10 mg / mL, 180 mmol / L, 0.3 mg / mL and 10 mmol / L, and the pH was adjusted to 4.2 to obtain the original liquid preparation of recombinant human urokinase for injection.
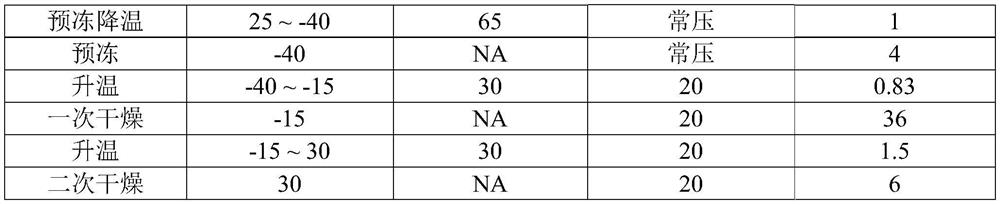
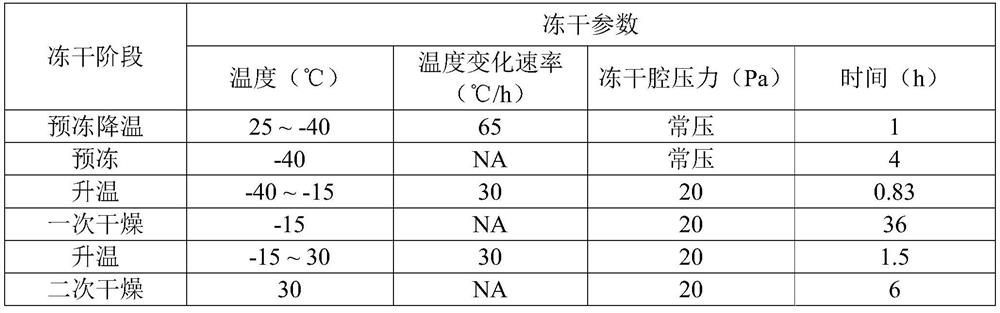
[0048] (2) place the obtained recombinant human urokinase original liquid preparation for injection on the bottom area to be 7cm 2 In the container of 5.4mL, it was then transferred to a freeze dryer for freeze-drying according to the following process to obtain the freeze-dried preparation of recombinant human pro-urokinase for injection.
[0049]
...
Embodiment 2
[0052] The present embodiment provides a liquid preparation of recombinant human urokinase for injection and a freeze-dried preparation, and the preparation method is as follows:
[0053] (1) Weigh recombinant human prourokinase, trehalose, polysorbate 80, citric acid / sodium citrate and mix and dissolve with water for injection, so that recombinant human prourokinase, trehalose, polysorbate 80, citrate The concentrations of acid / sodium citrate were respectively 8 mg / mL, 150 mmol / L, 0.2 mg / mL and 8 mmol / L, and the pH was adjusted to 4.0 to obtain the original liquid preparation of recombinant human urokinase for injection.
[0054] (2) place the obtained recombinant human urokinase original liquid preparation for injection on the bottom area to be 7cm 2 In a container of 5.0 mL, it is then transferred to a freeze dryer for freeze drying according to the following process to obtain the freeze-dried preparation of recombinant human pro-urokinase for injection.
[0055]
Embodiment 3
[0057] The present embodiment provides a liquid preparation of recombinant human urokinase for injection and a freeze-dried preparation, and the preparation method is as follows:
[0058] (1) Weigh recombinant human prourokinase, trehalose, polysorbate 80, citric acid / sodium citrate and mix and dissolve with water for injection, so that recombinant human prourokinase, trehalose, polysorbate 80, citrate The concentrations of acid / sodium citrate were respectively 12 mg / mL, 200 mmol / L, 0.4 mg / mL and 12 mmol / L, and the pH was adjusted to 4.4 to obtain the original liquid preparation of recombinant human urokinase for injection.
[0059] (2) place the obtained recombinant human urokinase original liquid preparation for injection on the bottom area to be 7cm 2 In a container of 4.5 mL, the filling volume is 4.5 mL, and then transferred to a freeze-drying machine for freeze-drying according to the following process to obtain the freeze-dried preparation of recombinant human pro-uroki...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Bottom area | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com