A kind of purification method of recombinant human urokinase
A technology of prourokinase and purification method, which is applied in the direction of biochemical equipment and methods, enzymes, peptidases, etc., can solve problems such as low safety, human hazards, low protein purity, and DNA exogenous pollution, and achieve improved The effect of safety, improving purity and reducing the risk of clinical application
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0097] Purification Step 1: Protein Capture
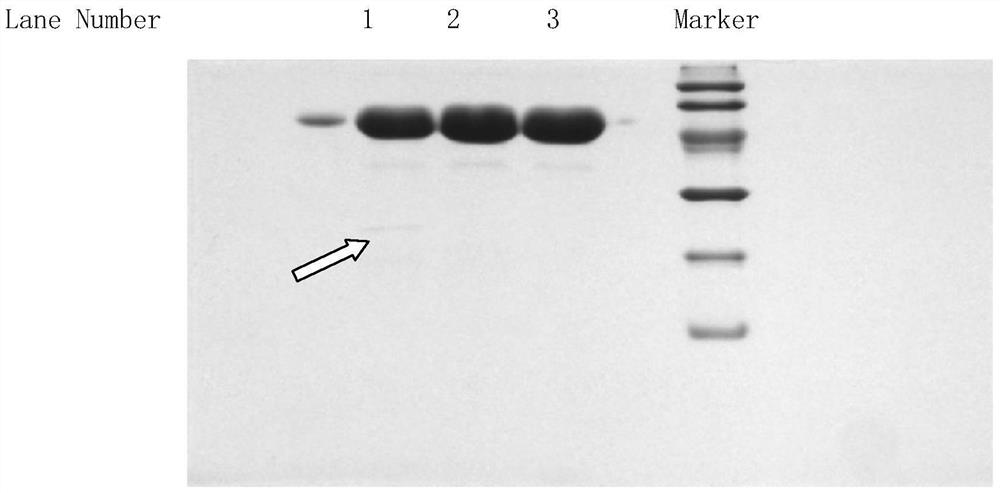
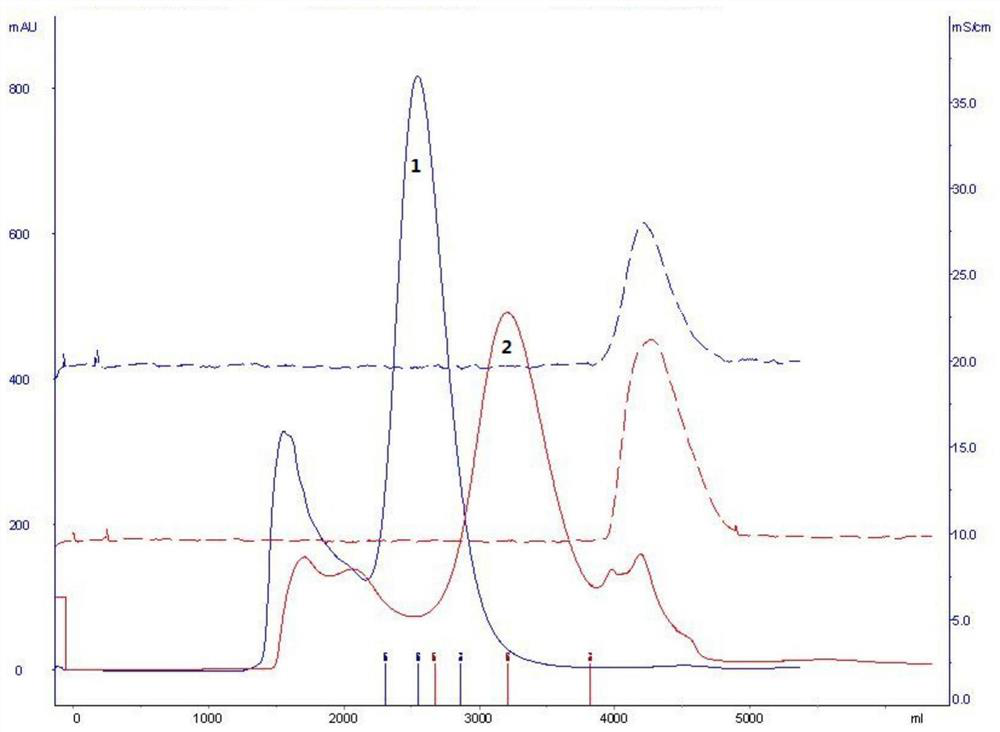
[0098] Exchange chromatography was carried out with Streamline SP cationic packing as the medium. The chromatographic column is equilibrated with 0.01mol / L phosphate buffer (pH 6.0 containing 0.1mol / L NaCl), and the supernatant of the fermentation broth is adjusted to pH 6.0 before loading the sample, and 0.01mol / L phosphate buffer is used (pH6.0 containing 0.5mol / L NaCl) elution, according to the UV 280 ultraviolet absorption curve to collect the eluted protein peak, which is the intermediate of Purification 1.
[0099] Purification Step 2: Gel Chromatography
[0100] Gel chromatography was carried out with Sephacryl S-200HR gel molecular sieve filler as the medium. The chromatographic column was equilibrated with 0.01mol / L phosphate buffer (pH6.0 containing 0.09mol / L NaCl), and the purified 1 intermediate was directly loaded with 0.01mol / L phosphate buffer (pH6.0 containing 0.09 mol / L NaCl), and collect the target protein peak...
Embodiment 2
[0110] Purification Step 1: Protein Capture
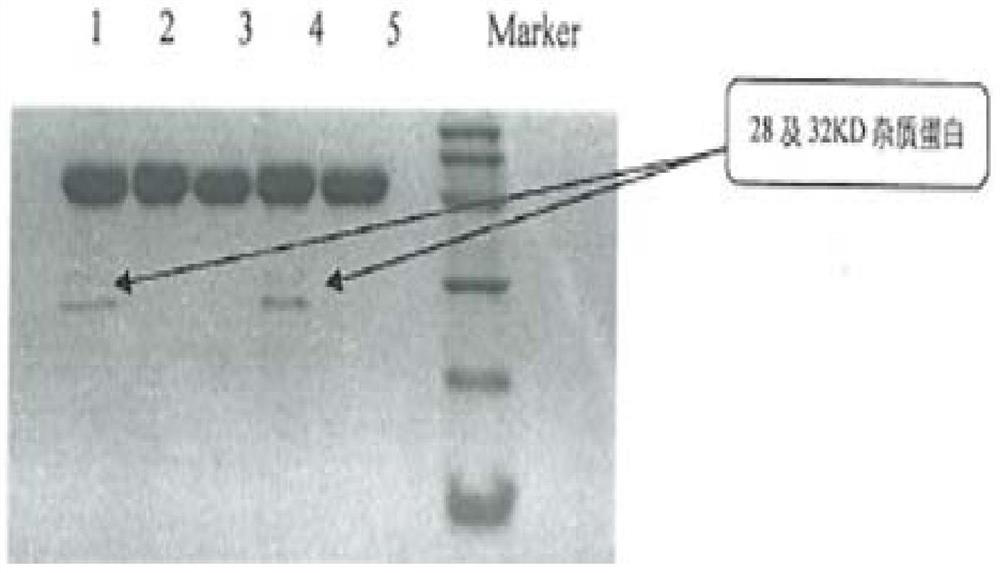
[0111] Exchange chromatography was carried out with Streamline SP cationic packing as the medium. The chromatographic column is equilibrated with 0.005mol / L phosphate buffer (pH5.5 containing 0.05mol / L NaCl), and the supernatant of the fermentation broth is adjusted to pH 5.5 before loading the sample, and 0.005mol / L phosphate buffer is used (pH 5.5 containing 0.3mol / L NaCl elution, according to the UV 280 ultraviolet absorption curve to collect the eluted protein peak, which is the intermediate of Purification 1.
[0112] Purification Step 2: Gel Chromatography
[0113]Gel chromatography was carried out with Sephacryl S-200HR gel molecular sieve filler as the medium. The chromatographic column was equilibrated with 0.005mol / L phosphate buffer (pH5.5 containing 0.05mol / L NaCl), and the purified 1 intermediate was directly loaded with 0.005mol / L phosphate buffer (pH5.5 containing mol / LNaCl), and collect the target protein peak ac...
Embodiment 3
[0123] Purification Step 1: Protein Capture
[0124] Exchange chromatography was carried out with Streamline SP cationic packing as the medium. The chromatographic column was equilibrated with 0.015mol / L phosphate buffer (pH6.5 containing 0.15mol / L NaCl), and the supernatant of the fermentation broth was adjusted to pH 6.5 before loading the sample, and 0.015mol / L phosphate buffer was used (pH 6.5 containing 0.7mol / L NaCl elution, according to the UV 280 ultraviolet absorption curve to collect the eluted protein peak, which is the intermediate of Purification 1.
[0125] Purification Step 2: Gel Chromatography
[0126] Gel chromatography was carried out with Sephacryl S-200HR gel molecular sieve filler as the medium. The chromatographic column was equilibrated with 0.015mol / L phosphate buffer (pH6.5 containing 0.12mol / L NaCl), and the purified 1 intermediate was directly loaded with 0.015mol / L phosphate buffer (pH6.5 containing 0.12 mol / L) elution, according to the UV 280 u...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com