Method for detecting purity of recombinant human prourokinase
A technology of pro-urokinase and detection method, applied in the field of detection and analysis, can solve the problems of poor separation effect of monomers and polymers, complex composition of finished preparations, and difficulty in detecting pro-urokinase polymers, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
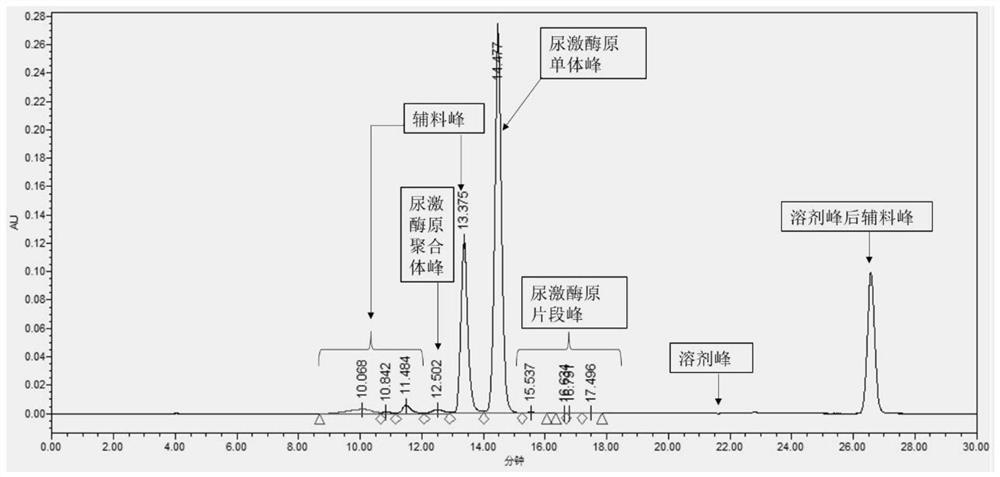
[0065] This embodiment provides a method for detecting the purity of recombinant human pro-urokinase finished product, said detection method comprising the following steps:
[0066] (1) Preparation of sample solution: add the recombinant human prourokinase finished product into water to dissolve, prepare a sample solution with a prourokinase protein concentration of 1 mg / mL, centrifuge and take the supernatant, which is the sample solution;
[0067] (2) SEC-HPLC for detection: use Waters ACQUITYArc high performance liquid chromatography and ultraviolet detector for detection, the specific conditions are as follows:
[0068] Mobile phase A: 0.1vol% acetic acid aqueous solution containing 100mmol / L sodium chloride (pH is 3.1);
[0069] Mobile phase B: acetonitrile;
[0070] SEC-HPLC column: Waters XBridge TM BEH Protein SEC (7.8mm×300mm, 2.5μm, );
[0071] Test parameters: flow rate 0.5mL / min, detection wavelength 280nm, elution time 30min, injection volume 30μL per needle...
Embodiment 2
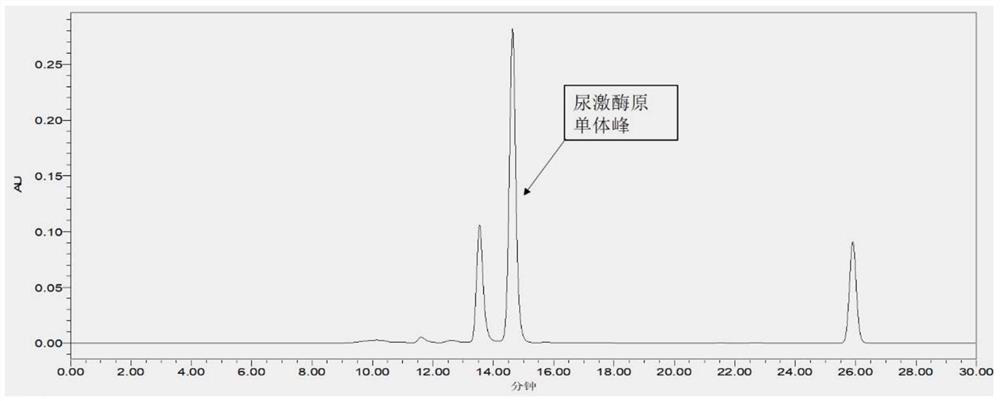
[0078] The present embodiment provides a method for detecting the purity of the original stock solution of urokinase, the detection method comprising the following steps:
[0079] (1) Preparation of sample solution: add the original urokinase solution into water to dissolve, prepare a sample solution with a concentration of 1 mg / mL of the original urokinase protein, centrifuge and take the supernatant, which is the sample solution;
[0080] (2) SEC-HPLC for detection: use Waters ACQUITYArc high performance liquid chromatography and ultraviolet detector for detection, the specific conditions are as follows:
[0081] Mobile phase A: 0.1vol% acetic acid aqueous solution containing 100mmol / L sodium chloride (pH is 3.1);
[0082] Mobile phase B: acetonitrile;
[0083] SEC-HPLC column: Waters XBridge TM BEH Protein SEC (7.8mm×300mm, 2.5μm, );
[0084] Test parameters: flow rate 0.5mL / min, detection wavelength 280nm, elution time 30min, injection volume 30μL per needle, set tem...
Embodiment 3
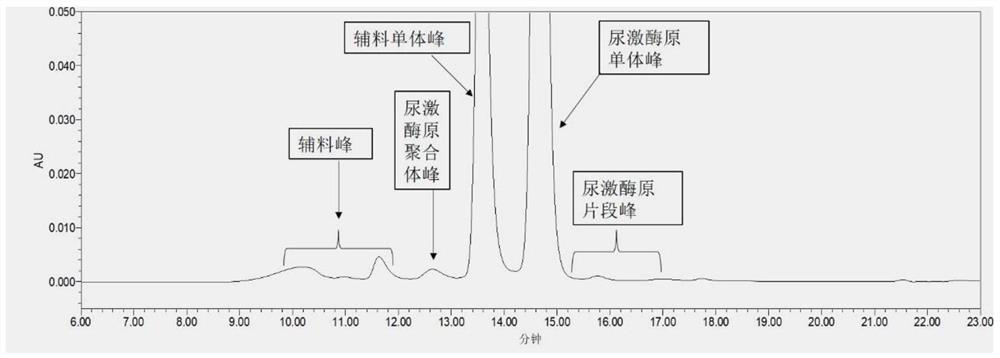
[0090]This embodiment provides a method for detecting the purity of recombinant human prourokinase finished product. The only difference from Example 1 is that mobile phase A is 0.1vol% TFA aqueous solution containing 100mmol / L sodium chloride, and NaOH is added to adjust the pH to 3.1, other test steps are the same as in Example 1;
[0091] The specific test results are shown in Table 3 below:
[0092] table 3
[0093] mobile phase A pH Aggregate and monomer resolution p / v 0.1% TFA, 100mM NaCl(NaOH) 3.1 3.6
[0094] From the comparison of Example 1 and Example 3, it can be seen that when the finished product of recombinant human urokinase is used as the test sample, if adding trifluoroacetic acid, it is necessary to add an appropriate amount of NaOH to adjust the pH to 3.1, and using acetic acid, not only the degree of separation can be further improved , and do not need to adjust the pH, easy to operate.
PUM
| Property | Measurement | Unit |
|---|---|---|
| wavelength | aaaaa | aaaaa |
| wavelength | aaaaa | aaaaa |
| length | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com