Method for purification and virus removal of recombinant human prourokinase
A pro-urokinase, virus technology, applied in biochemical equipment and methods, enzymes, peptidases, etc., can solve the problems of low protein purity, viral exogenous pollution, low safety, and inability to be used in the human body.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
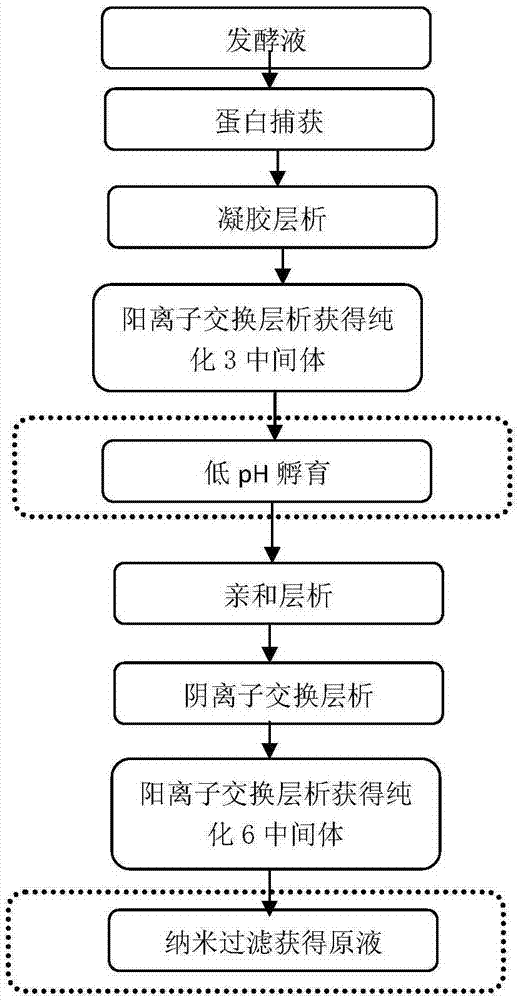
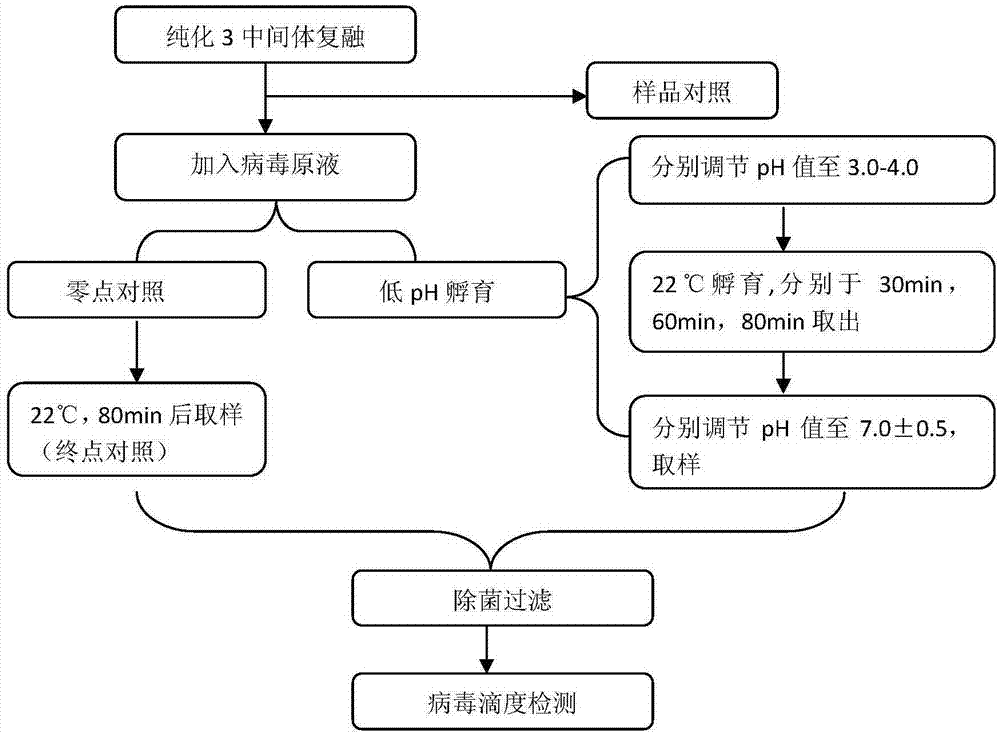
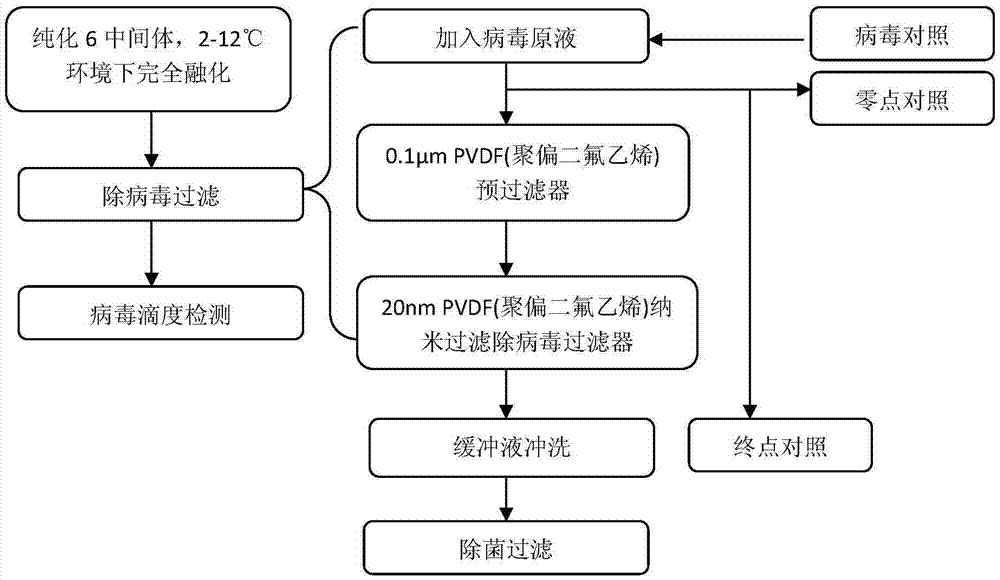
[0197] Purification step 1: protein capture
[0198] Exchange chromatography with Streamline SP cation packing as the medium. The chromatography column is balanced with 0.01mol / L phosphate buffer (pH 6.0 contains 0.1mol / L NaCl), and the supernatant is taken from the fermentation broth to adjust the pH to 6.0 and then loaded, using 0.01mol / L phosphate buffer (pH6.0 contains 0.5mol / L NaCl) elution, collect the eluted protein peak according to the UV 280 ultraviolet absorption curve, which is the purified intermediate 1.
[0199] Purification step 2: Gel chromatography
[0200] Gel chromatography was performed with Sephacryl S-200 HR gel molecular sieve filler as the medium. The chromatography column was balanced with 0.01mol / L phosphate buffer (pH6.0 containing 0.09mol / L NaCl), the purified intermediate 1 was directly loaded, and 0.01mol / L phosphate buffer (pH6.0 containing 0.09 mol / L NaCl) and collect the target protein peak according to the UV 280 ultraviolet absorption curve, whi...
Embodiment 2
[0214] Purification step 1: protein capture
[0215] Exchange chromatography with Streamline SP cation packing as the medium. The chromatographic column is balanced with 0.005mol / L phosphate buffer (pH5.5 containing 0.05mol / L NaCl), and the supernatant is taken from the fermentation broth to adjust the pH to 5.5 and then loaded, using 0.005mol / L phosphate buffer (pH5.5 contains 0.3mol / L NaCl elution, collect the eluted protein peak according to the UV 280 ultraviolet absorption curve, which is the purified intermediate 1.
[0216] Purification step 2: Gel chromatography
[0217] Sephacryl S-200HR gel molecular sieve packing was used for gel chromatography. The column is balanced with 0.005mol / L phosphate buffer (pH5.5 containing 0.05mol / L NaCl), the purified intermediate 1 is directly loaded, and 0.005mol / L phosphate buffer (pH5.5 containing 0.05 mol / LNaCl) and collect the target protein peak according to the UV 280 ultraviolet absorption curve, which is the purified intermediate ...
Embodiment 3
[0231] Purification step 1: protein capture
[0232] Exchange chromatography with Streamline SP cation packing as the medium. The chromatographic column is balanced with 0.015mol / L phosphate buffer (pH6.5 contains 0.15mol / L NaCl), and the supernatant is taken from the fermentation broth to adjust the pH to 6.5 and then loaded, using 0.015mol / L phosphate buffer (pH6.5 contains 0.7mol / L NaCl elution, collect the eluted protein peak according to the UV 280 ultraviolet absorption curve, which is the purified intermediate 1.
[0233] Purification step 2: Gel chromatography
[0234] Sephacryl S-200HR gel molecular sieve packing was used for gel chromatography. The chromatography column was balanced with 0.015mol / L phosphate buffer (pH6.5 containing 0.12mol / L NaCl), and the purified intermediate 1 was directly loaded with 0.015mol / L phosphate buffer (pH6.5 containing 0.12mol / L NaCl). mol / L) and collect the target protein peak according to the UV 280 ultraviolet absorption curve, which is...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com