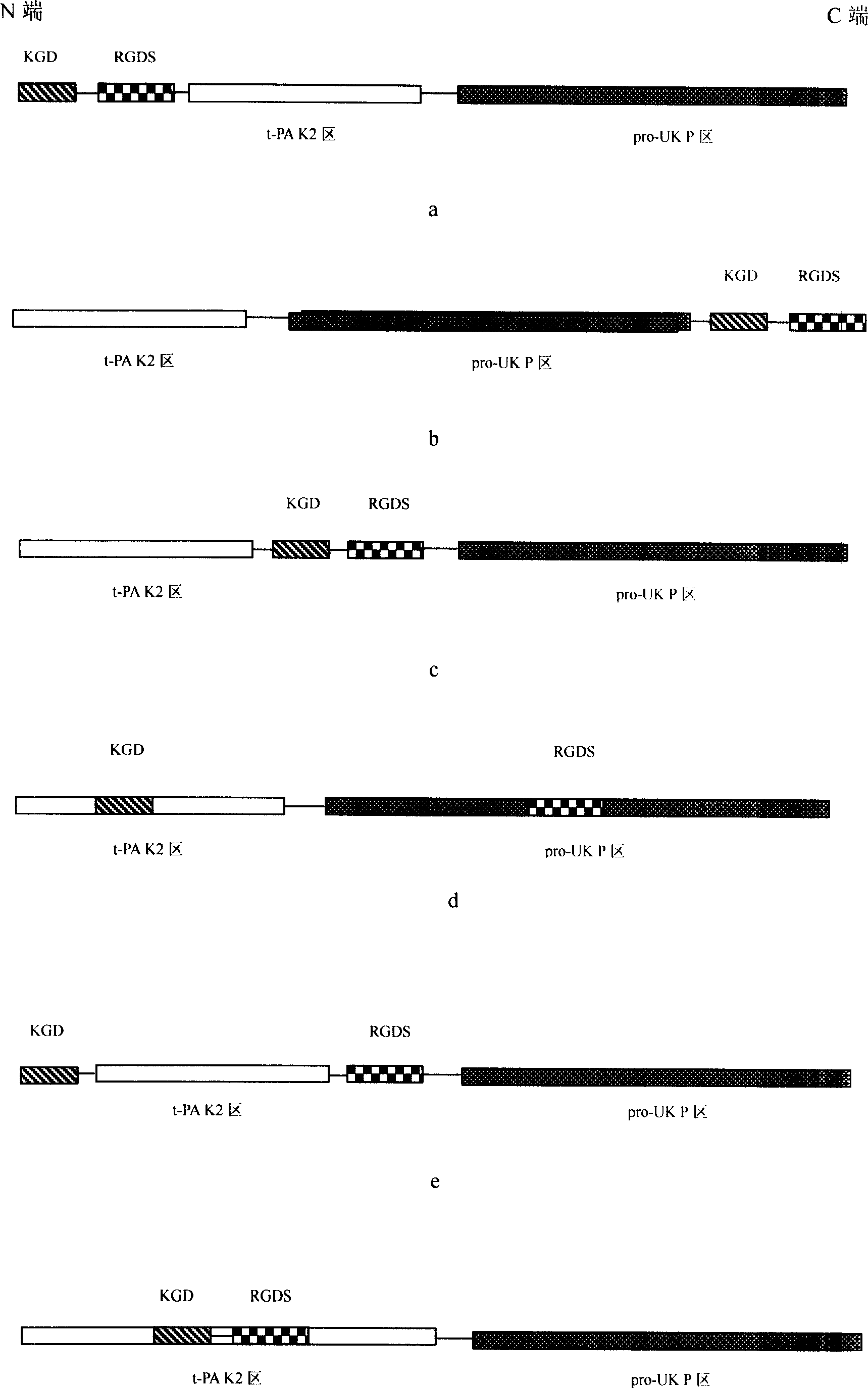
Prourokinase modifier, preparation method, pharmaceutical composition, use, encoding gene, carrier containing gene and transformation cell
A kind of technology of prourokinase and modified body, applied in the field of genetic engineering
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0023] prourokinase variant M 2 Gene construction (see the sequence listing SEQ ID NO.1 and SEQ ID NO.2 for nucleotide and amino acid sequences respectively)
[0024] 1. t-PA K 2 district construction
[0025] According to literature (Pennica, D., et al.Cloning and expression of human tissue-type plasminogenactivator cDNA in E.coli.Nature 301(5897), 214-221(1983).), K 2 The full sequence of zones is:
[0026] GTGACTGCTACTTTGGGAATGGGTCAGCCTACCGTGGCACGCACAGCCTCCACCGAG
[0027] TCGGGTGCCTCCTGCCTCCCGTGGAATTCCATGATCCTGATAGGCAAGGTTTACAC
[0028] AGCACAGAACCCCAGTGCCCAGGCACTGGGCCTGGGCAAACATAATTACTGCCGG
[0029] AATCCTGATGGGGATGCCAAGCCCTGGTGCCACGTGCTGAAGAACCGCAGGCTGA
[0030] CGTGGGAGTACTGTGATGTGCCCTCCTGCT
[0031] Synthesize K in three stages 2 template:
[0032] first paragraph:
[0033] AAGTGGATATCATGTGCTACTTTGGGAATGGGTCAGCCTACCGTGGCACGCAC
[0034] AGCCTCACCGAGTCGGGTGCCTCCTGCCTGCCG TGGAATTCCATGAT
[0035] The upstream primer is: 5'-GGATATCATGTGCTACTTTGGGAA-3' restriction...
Embodiment 2
[0076] Containing Prourokinase Modified M 2 Expression in Chinese hamster ovary cells
[0077] 1. t-PA K 2 Region construction: according to the method of Example 1.
[0078] 2. ProUK-P region construction: according to the method of Example 1.
[0079] 3. Construction of Signal+KGD+RGDS guide peptide region
[0080] Synthesize a single-stranded DNA fragment containing the Signal+KGD+RGDS sequence as a peptide guide template
[0081] GAAGCTTATGAGAGCCCTGCTGGCGCGCCTGCTTCTCTGCGTCCTGGTCCTGAGCG
[0082] ACTCCAAAGGCCGCGGCGACAGCAAGGGTGACTGGGATATCC
[0083] The upstream primer is: 5'-GAAGCTTATGAGAGCCCTGCTG-3' restriction site is HindIII
[0084] The downstream primer is: 5'-AGATATCCCAGTCACCCTTGCT-3' restriction site is EcoRV
[0085] The PCR amplification product is the Signal+KGD+RGDS guide peptide. After the amplified product was recovered and purified, it was ligated with the pGEM-Teasy vector and transformed into E.coli DH5α.
[0086] 4. pcDNA-M 2 Plasmid construction
...
Embodiment 3
[0116] Expression of Transformant M Using Insect Baculovirus Expression System 2
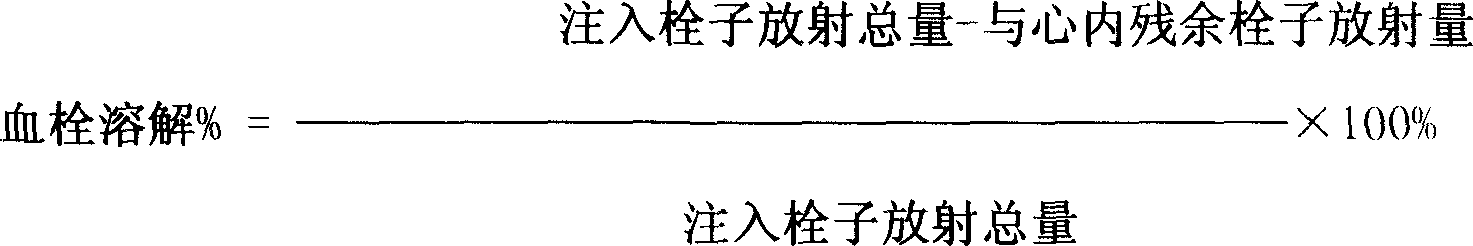
[0117] M 2 The gene was cloned into the insect baculovirus expression vector pFast-BacHTa, transfected into Sf9 insect cells through liposome Superfect, harvested the culture supernatant, passed through the affinity chromatography column affinity layer coupled with anti-prourokinase antibody After analysis, it was desalted and concentrated, and freeze-dried to obtain the purified prourokinase variant M 2 protein powder. SDS-PAGE electrophoresis identified that the molecular weight of the pro-urokinase variant M2 expressed in Sf9 insect cells was in line with expectations.
[0118] The affinity chromatography column coupled with anti-prourokinase antibody was provided by Shanghai Sangon Bioengineering Technology Service Co., Ltd., and the transfection method was referred to the "Molecular Cloning Experiment Guide" (Sambrool, J. et al., Huang Peitang et al. Translated, Molecular Cloning Experi...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Molecular weight | aaaaa | aaaaa |
| Molecular weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com