Method for detecting poloxamer residual quantity in recombinant human urokinase raw material for injection
A detection method, poloxamer technology, applied in the field of detection and analysis, can solve problems such as increasing the difficulty of optimization and difficult separation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0089] Example 1: A method for detecting the residual amount of poloxamer in the raw material of recombinant human urokinase for injection
[0090] Sample pretreatment method: Add 200 μl of original sample to 300 μl of acetonitrile that has been frozen overnight, shake for 30 seconds, sonicate for 3 minutes, shake again for 30 seconds, centrifuge at 9500 rpm for 1 minute, and take the supernatant.
[0091] Standard solution preparation method: Take poloxamer standard, weigh and prepare standard solution, the concentration is 2mg / ml, 1mg / ml, 0.5mg / ml, 0.25mg / ml, 0.1mg / ml, 0.04mg / ml, 0.02mg / ml, 0.01mg / ml, 0.005mg / ml.
[0092] Chromatographic conditions (HPLC-CAD): column Acclaim TM Surfactant Plus column, 3μm, 30mm×150mm; injection volume 10μL; column temperature 40℃; flow rate 0.7mL / min; CAD detector: sampling frequency 5 Hz, peak filtration time parameter 3.6 seconds, nebulization temperature 50℃; mobile phase and gradient elution are shown in Table 1.
[0093]Detection: ...
Embodiment 2
[0094] Embodiment 2: verification of the method
[0095] 1. Preparation of samples and solutions:
[0096] 1.1 Standards and samples
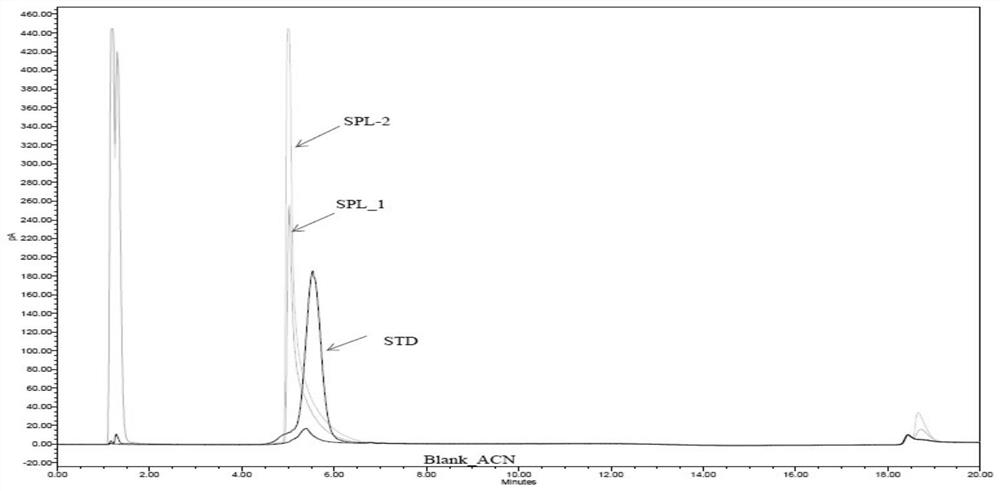
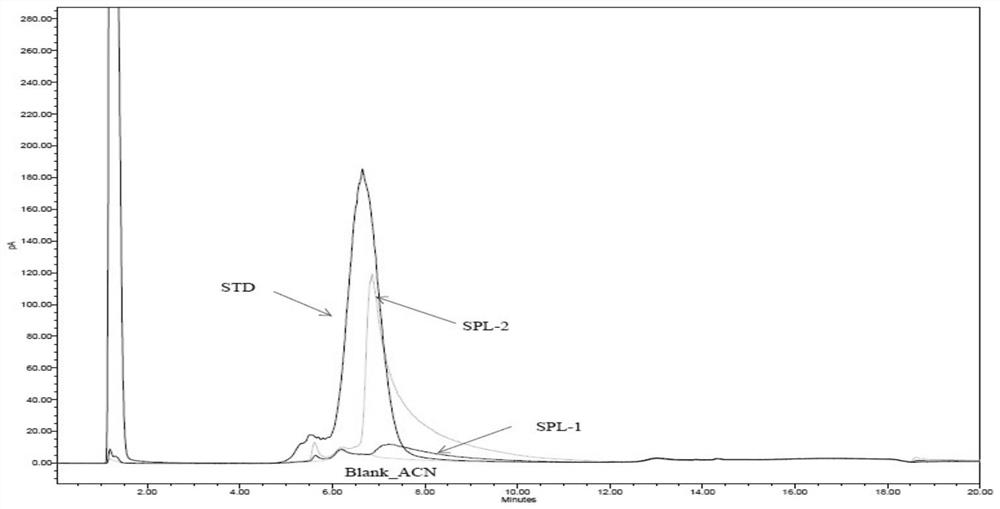
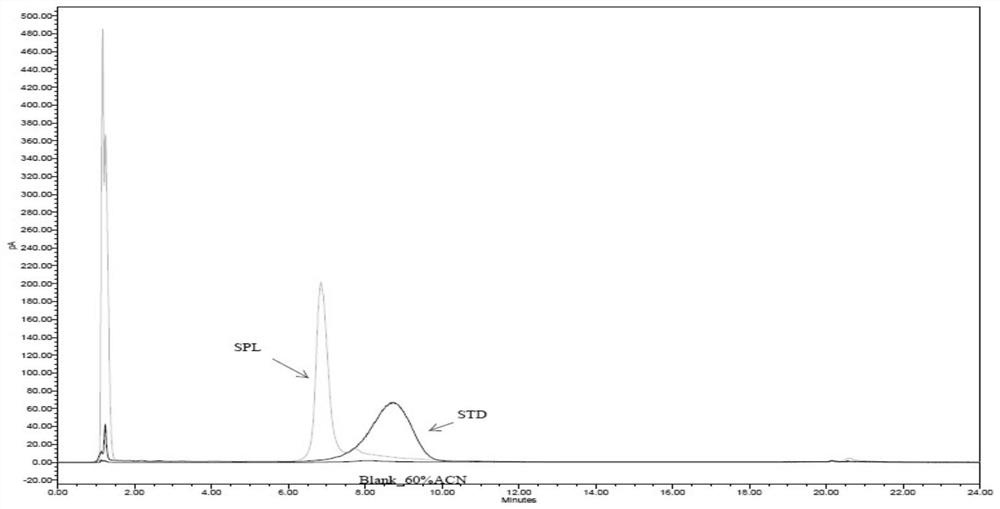
[0097] Standard product: F68 (STD), Sigma, batch number SLBP3447V;
[0098] Sample 1: recombinant human urokinase stock solution for injection (SPL1), Shanghai Tasly, batch number T20161219B;
[0099] Sample 2: Recombinant human pro-urokinase stock solution for injection (SPL2), Shanghai Tasly, batch number V20170526B.
[0100] Blank sample: a sample not containing F68 and recombinant human prourokinase, 60% acetonitrile (Blank).
[0101] 1.2 Preparation of sample solution
[0102] Stock#1 solution (2mg / mL): Accurately weigh 10.84mg of F68 into a 5mL volumetric flask, add 3mL of diluent, dilute to the mark with diluent, and mix well;
[0103] Stock#2 solution (2mg / mL): Accurately weigh 10.38mg of F68 into a 5mL volumetric flask, add 3mL of diluent, dilute to the mark with diluent, and mix well;
[0104] L8 solution (2mg / mL): take stock#2 ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com