A preparation process for separating and purifying recombinant human pro-urokinase from recombinant Escherichia coli fermentation broth
A technology for recombining Escherichia coli and prourokinase, which is applied in the field of preparation technology of recombinant human prourokinase, can solve the problems of low protein recovery efficiency and purity, and achieves a unique thrombolytic mechanism, no or low toxic and side effects, and high output. high effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
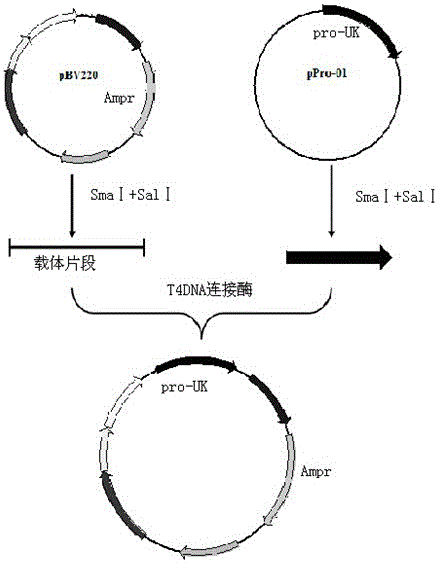
[0029] 1. The prokaryotic expression vector pBV220-proUK of human prourokinase was constructed.
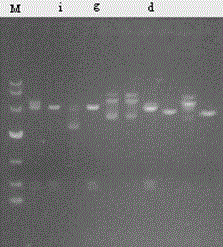
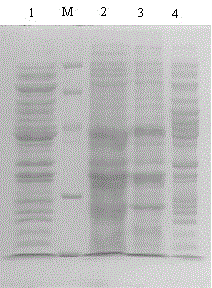
[0030] The proUK-containing gene obtained by PCR was cut with SmaI and SalI, and then ligated with the vector pBV220 cut with SmaI and SalI; the engineering strain DH5α-pBV220-proUK was constructed; the prokaryotic expression vector pBV220 containing human prourokinase was constructed -proUK (eg figure 1 , figure 2 shown).
[0031] 2. Fermentation and high-efficiency expression of recombinant human prourokinase, specifically:
[0032] Activation, fermentation, induction and recovery of inclusion bodies of engineered bacteria: inoculate engineered strains stored at -70°C on nutrient agar plate medium containing Amp, and culture at 37°C for 14-16 hours; pick a single colony and inoculate into Amp-containing In LB liquid medium, culture on a shaking table at 32°C for more than 12 hours; inoculate the bacterial liquid into the fermentation medium containing Amp at a ratio of 1:1...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com