Anthracene ring triazole compound, and preparation method and application thereof
A technology for triazoles and compounds, which is applied in the field of organic synthesis, can solve the problems that have not been found or retrieved, and achieve the effects of simple and easy reaction operation, large profit margins, and high purity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0030] The molar ratio of 9,10-dibromoanthracene: triazole: potassium carbonate: copper oxide is 2:10:30:1
[0031] CuO (0.0398 mg, 0.5 mmol), potassium carbonate (2.0731 g, 15 mmol), triazole (0.345 mg, 5 mmol), and 9,10-Dibromoanthracene (0.3360 g, 1 mmol), 20 mL DMF. Start stirring at 100 o C, reacted for 24 hours. After the reaction, the reaction solution was lowered to room temperature, filtered, and 100 mL of water was added to the filtrate, a large amount of precipitate was precipitated, filtered with suction, and the filter cake was collected, with a yield of 60%.
Embodiment 2
[0033] Preparation:
[0034] The molar ratio of 9,10-dibromoanthracene: triazole: potassium carbonate: copper oxide is 2:10:30:1
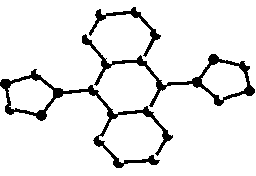
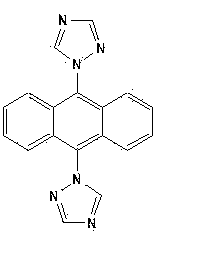
[0035] CuO (0.0398 mg, 0.5 mmol), potassium carbonate (2.0731 g, 15 mmol), triazole (0.345 mg, 5 mmol), and 9,10-Dibromoanthracene (0.3360 g, 1 mmol), 20 mL DMF. Start stirring at 150 o C, reacted for 60 hours. After the reaction, the reaction solution was lowered to room temperature, filtered, and 100 mL of water was added to the filtrate, and a large amount of precipitate was precipitated, filtered with suction, the filter cake was collected, and recrystallized with water to obtain 1-[9-(1H-1,2,4-triazol Azol-1-yl)anthracen-10-yl]-1H-1,2,4-triazole single crystal, yield 60%.
[0036] The crystal structure was determined using an APEX II CCD area detector, using graphite monochromatized Mokα rays (l = 0.71073 ?) as the incident radiation, and w - 2q Diffraction points were collected by scanning, and unit cell parameters were corrected by le...
Embodiment 3
[0041] Application of 1-[9-(1H-1,2,4-triazol-1-yl)anthracen-10-yl]-1H-1,2,4-triazole single crystal in preparation of luminescent material.
[0042] Method: The excitation wavelength and emission wavelength of the compound (Example 1) were scanned by a fluorescence spectrophotometer, and the optimal wavelength was selected and determined.
[0043] Conclusion: The excitation and emission wavelengths of this compound are 365 nm and 558 nm, respectively.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com