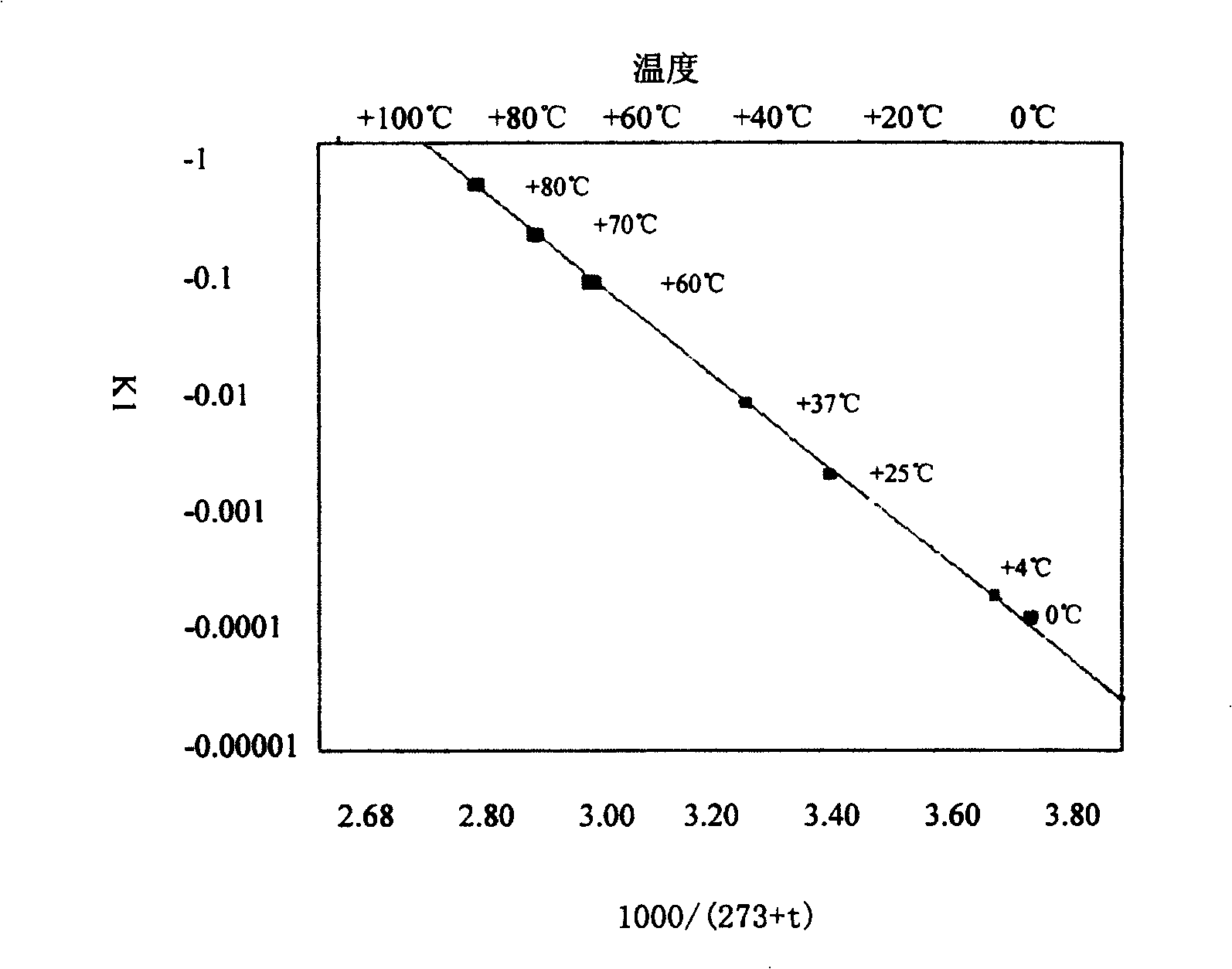
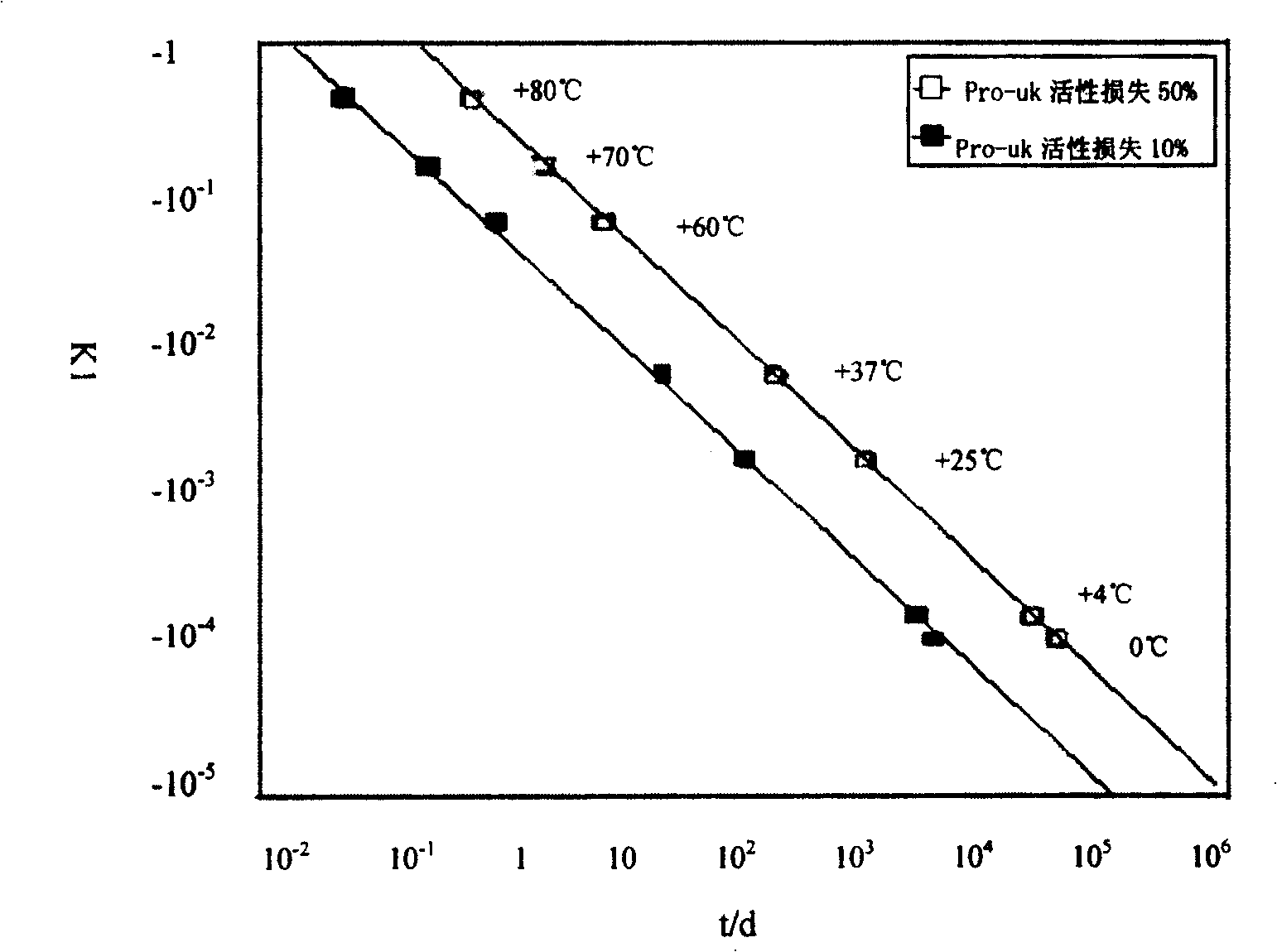
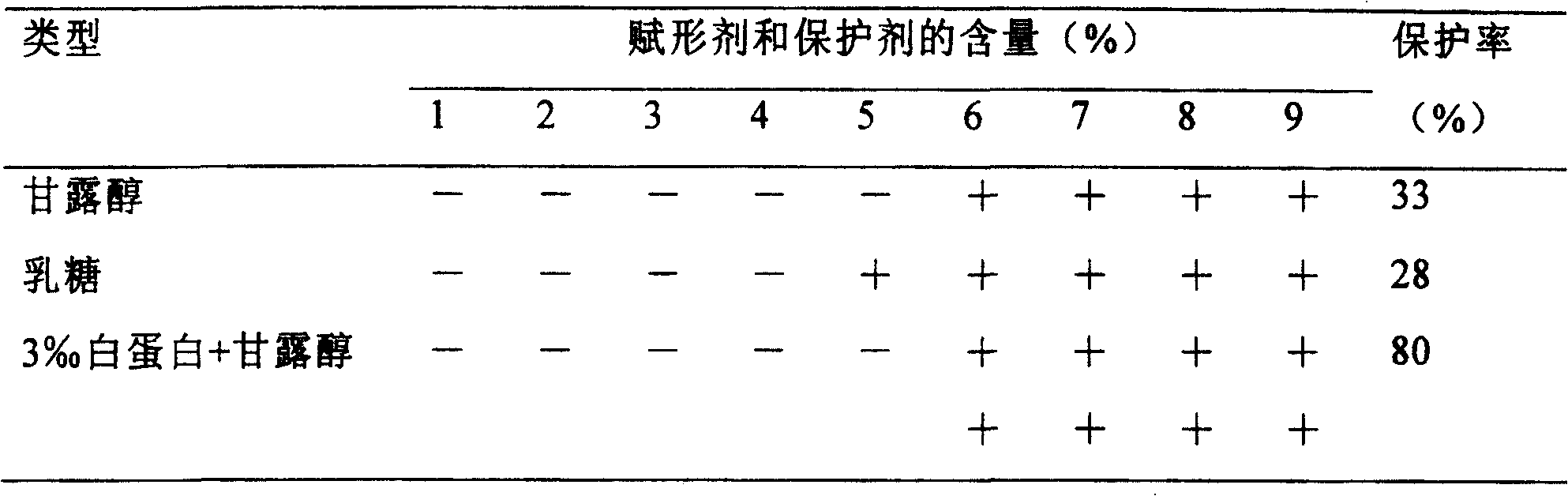
Composition containing active prourokinase, freeze-drying process and freeze-dried preparation thereof
A technology of pro-urokinase and freeze-dried preparations, which is applied in the field of medicine and can solve problems such as instability of pro-urokinase preparations
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0018] Preparation of prourokinase
[0019] (See Chinese patent: preparation method of recombinant human glycosylated pro-urokinase, announcement number CN1062016C, announcement date: February 14, 2001)
[0020] (1) Cloning of prourokinase gene and construction of expression vector
[0021] Detroit 562 cells were induced by myristate (PMA), total RNA was extracted, and a cDNA library was constructed. A positive clone containing a gene fragment encoding urokinase was obtained by screening, and the full-length cDNA gene of human prourokinase was obtained. Gene recombination technology operation, the two transcription units expressing prourokinase and dihydrofolate reductase genes are placed in the same vector, which are controlled by metallothionein (MT) and SV40 early promoters respectively.
[0022] (2) Transfection and screening of highly expressed CHO engineered cells
[0023] Transfect 20-40 μg pMTSV-du plasmid DNA into CHO-dhfr by calcium phosphate coprecipitation method...
Embodiment 2
[0029] Vacuum Freeze Drying of Pro-uk Compositions
[0030] Purified Pro-uk pure product 2mg / ml, add mannitol to 6% (weight / volume ratio), albumin to 3‰ (weight / volume ratio), phosphate buffer concentration to 10mmol / L, chlorine The concentration of sodium chloride is 0.05mol / L, and it is divided into 1ml ampoules, put into a lyophilizer pre-cooled to -40°C, pre-frozen for 3 hours, and then vacuumized, and the temperature is raised to -30°C for the first time. After the internal moisture is basically sublimated, the temperature is raised for the second time to make the temperature rise to 30°C, and after stabilization, it is kept for 1 hour, and then the freeze-drying can be ended.
Embodiment 3
[0032] Vacuum Freeze Drying of Pro-uk Compositions
[0033] Purified Pro-uk pure product 4mg / ml, add mannitol to 6% (weight / volume ratio), albumin to 3‰ (weight / volume ratio), phosphate buffer concentration to 10mmol / L, chlorine The concentration of sodium chloride is 0.10mol / L, and it is divided into 1ml ampoules, placed in a freeze dryer that has been cooled to -40°C in advance, pre-frozen for 3 hours, and then vacuumized, and the temperature is raised to -30°C for the first time. After the internal moisture is basically sublimated, the temperature is raised for the second time to make the temperature rise to 30°C, and after stabilization, it is kept for 1 hour, and then the freeze-drying can be ended.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com