Biodegradable Extravascular Stent
a biodegradable, extravascular technology, applied in the direction of prosthesis, blood vessels, surgery, etc., can solve the problems of difficult control of fibrin glue use (e.g. vein graft coating) and high failure rate of aorta-coronary and peripheral vein grafts
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
example 1
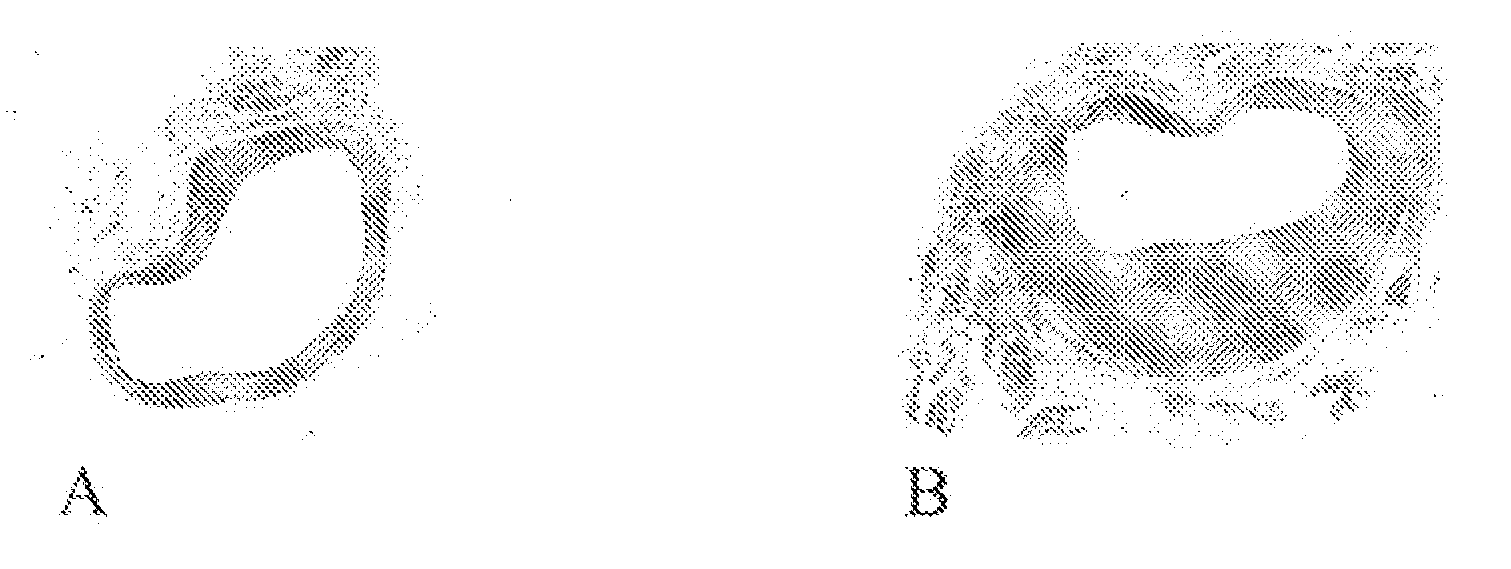
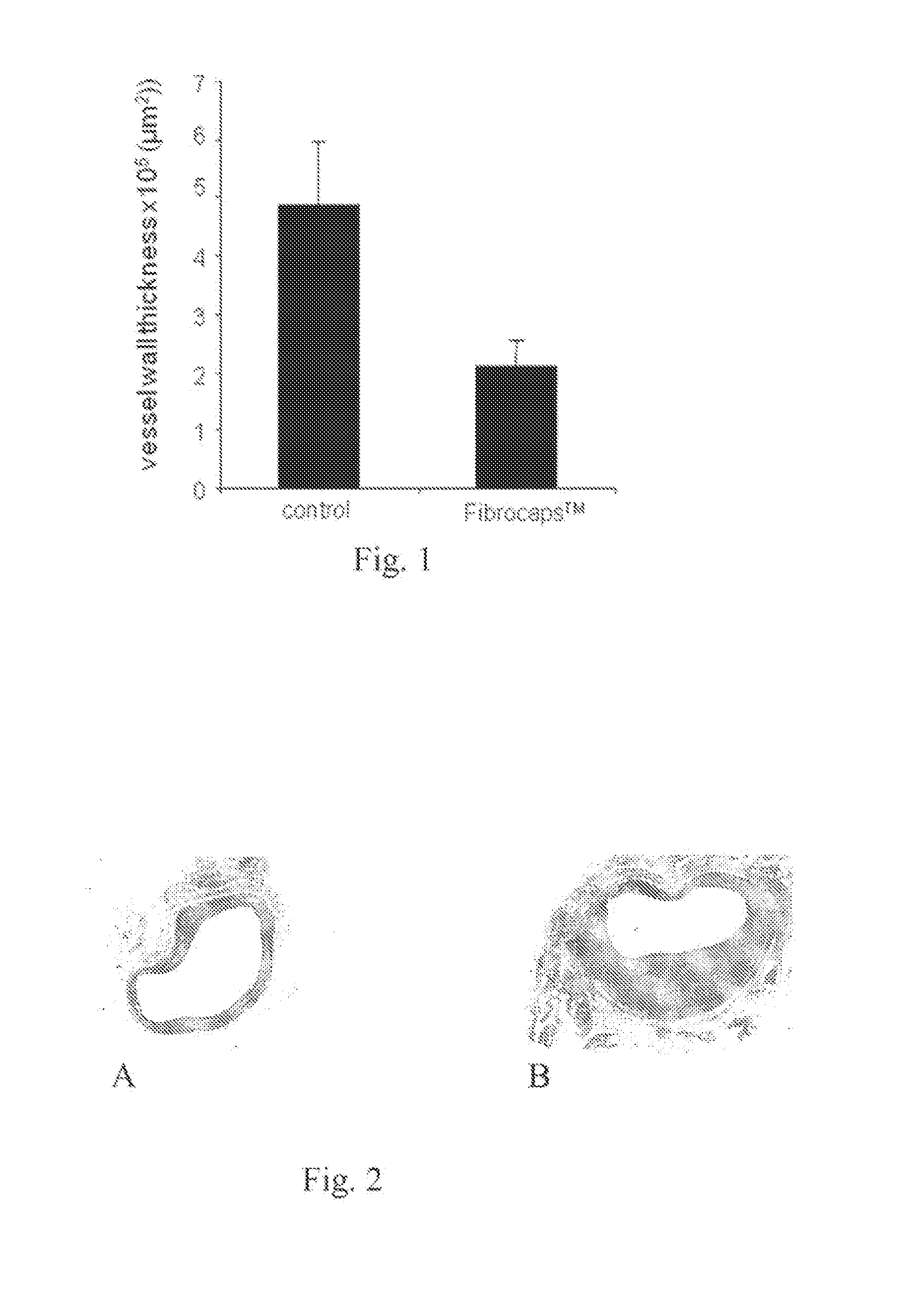
Testing of Fibrocaps for Extravascular Stent in a Mouse Vein Graft Model
[0023]Dry powder formulation Fibrocaps™, containing human plasma derived fibrinogen (6% w / w based on Fibrocaps) (ZLB. Marburg, Germany) and thrombin (500 IU / gram of Fibrocaps)(SNBTS, Glasgow, UK) was tested in transgenic mice that arc susceptible to cholesterol induced atherosclerosis, according to the method described by Lardenoye et al. (Circulation Res. (2002) Oct. 4, 577). In brief, the animals are fed a high-fat diet containing 0.5% cholate to improve intestinal cholesterol uptake and suppress bile-acid synthesis. This leads to increased plasma cholesterol levels.
[0024]After 4 weeks of chow or HFC 0.5%, mice are anesthetized with Hypnorm (Bayer, 25 mg / kg) and Dormicum (Roche, 25 mg / kg). The procedure used for vein grafts was similar to that described by Zou et al. (Am. J. Pathol. (1998) 153. 1301).
[0025]In brief, the right common carotid artery was dissected free from its surrounding from the bifurcation at...
PUM
| Property | Measurement | Unit |
|---|---|---|
| diameter | aaaaa | aaaaa |
| diameter | aaaaa | aaaaa |
| diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com