Compositions for use in cardioplegia comprising esmolol and adenosine
a technology of adenosine and esmolol, which is applied in the field of compositions for use in cardioplegia, can solve the problems of undesirable side effects of toxicity, drawbacks and complications, and application of prior art arresting agents such as lidocaine to patients at the required dose, and achieves no or minimal toxic effect, and clears quickly and reliably.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
Esmolol and Adenosine Combination
[0118]Esmolol and adenosine are rapidly inactivated by the red blood cell esterases and the blood vessel adenosine deaminase respectively. This makes the clearance of these agents fast and independent from the kidneys or the liver, contrary to most other pharmacological agents used as arresting agents in cardioplegia. This makes these two agents an effective combination to be used in a cardioplegic preparation such as a crystalloid preparation at high concentrations to arrest the heart and at the same time they can be cleared from the system rapidly and effectively as when the heart is ready to be started during cardiac surgery e.g. by washing out the drug with blood reperfusion.
[0119]We teach the use of Esmolol at concentrations higher than its p-blocking effect (0.01-0.1 mmol / L) to induce arrest and we add adenosine as a synergistic arresting agent in order to use lower amounts of esmolol, yet still surprisingly induce effective arrest. This combin...
example 2
Dose Determination
[0121]All the following experiments performed in a Langendorff perfused rat heart.
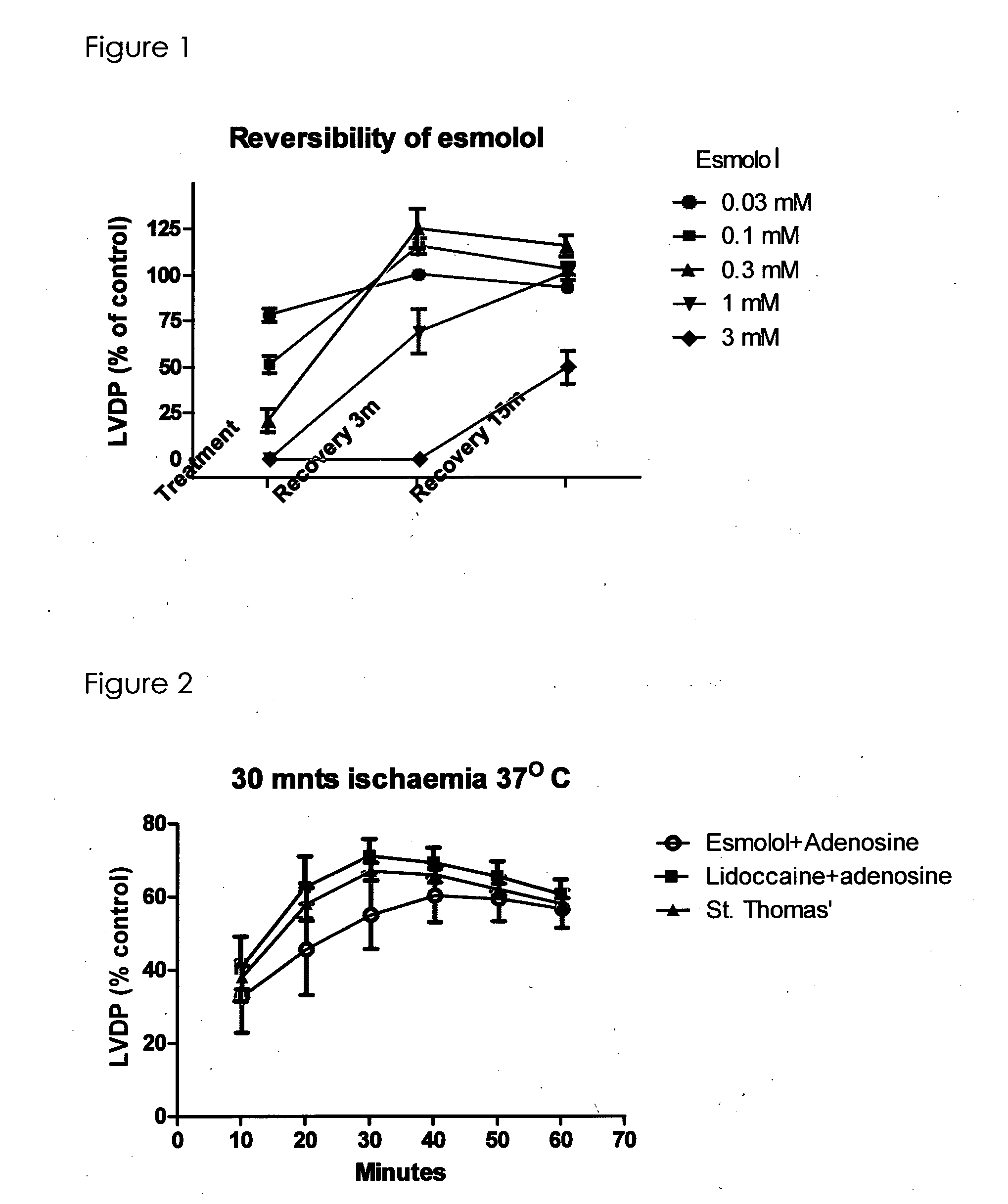
[0122]The first study is to determine the reversibility of esmolol at different concentrations after treatment with esmolol for 10 minutes then washout without ischaemia and LVDP was measured 9 minutes after starting the treatment and 3 minutes and 15 minutes after washing out the drug.
[0123]The following doses were studied 0.03, 0.1, 0.3, 1, 3 mM. (n=5 each group) 1 mM of esmolol was required to induce arrest but 0.3 mM had a better reversibility (LVDP recovery) profile (FIG. 1). It is therefore decided that esmolol concentration between 0.3 and 1 mM offers reasonable arresting effect with good reversibility profile.
example 3
Combination Dosing
[0124]Adenosine was added to esmolol (0.6 mM) at the following concentration: 0.125 mM, 0.25 mM, 1 mM. With normothermic (37° C.) 30 minutes ischaemia.
[0125]Esmolol 0.6 mM+adenosine 0.125 mM arrest time was prolonged cal (70 sec). (Table 1)
[0126]Esmolol 0.6 mM+adenosine 1 mM arrest time was fast but recovery was poor 6 to 40% (Table 2)
[0127]It was then decided to study adenosine at 0.25 mM which gives acceptable arresting time (50 seconds) with reasonable recovery
TABLE 1Arrest timeEsmololAdenosine(Sec)0.6 mM0.125 mM 700.6 mM0.25 mM 530.6 mM0.5 mM500.6 mM1.0 mM38
TABLE 2Examples of different concentrations of esmolol + Adenosine (30 minutes ischaemia at 37° C.)102030405060EsmololAdenosineBaselineminminminminminmin0.6 mM0.25 mM135455570777578LVDP291276275278279278274HR6605753515153EDP33.3340.7451.8557.0455.5657.78LVDP(% base)0.6 mM 1 mM156102037636462LVDP333240260290287274282HR4807063646165EDP6.4112.8223.7240.3841.0339.74LVDP(% base)0.6 mM0.125 mM 127182143616572LVDP...
PUM
| Property | Measurement | Unit |
|---|---|---|
| flow rate | aaaaa | aaaaa |
| flow rate | aaaaa | aaaaa |
| pH | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com