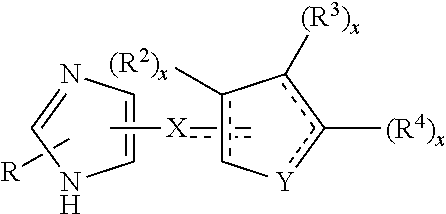
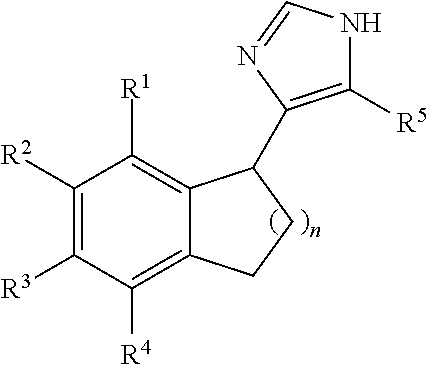
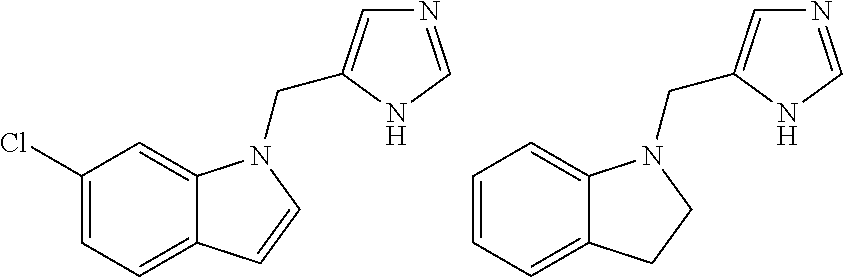
Reversed biaryl spiroaminooxazoline analogues as alpha2c adrenergic receptor modulators
a technology of adrenergic receptor and reverse biaryl spiroaminooxazoline, which is applied in the direction of biocide, drug composition, peptide/protein ingredients, etc., can solve the problems of undesirable side effects of compounds having adrenergic activity, such as 2a agonists, and achieve the effect of minimizing adverse side effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
Preparative Example 1
[0433]
Step 1
[0434]5-Bomo-tetralone 1A (30 g, 134 mmol) in DCM (200 mL) was added TMSCN (20 mL, 1.2 equiv. 160 mmol) at 0° C. followed by AlCl3 (3 g, 0.2 equiv., 26 mmol). The reaction was stirred at r.t. for 1 hr before been diluted with EtOAc (500 mL), washed with aq. NaOH (200 mL) and brine (100 mL). The organic layer was dried with Na2SO4, concentrated by rotavap and chromatographed (0% to 40% EtOAc / Hexane) to give the desired product 1B (37.4 g, 86%).
Step 2
[0435]Compound 1B (37.4 g, 116 mmol) in THF (300 mL) at 0° C. was added LAH (150 mL, 1.0M in Et2O, 150 mmol). The reaction was stirred at 0° C. for 0.5 hr before quenched by adding water dropwise until no gas was released: The reaction was then added aq. NaOH (1.0 M, 150 mL) and extracted with DCM thoroughly. The organic layer was separated, dried with Na2SO4 and concentrated. The crude (1C, 30 g, 99%) was used in the next step without further purification.
Step 3
[0436]A solution of 1C (1.3 g, 5.4 mmol) in ...
example 2
Preparative Example 2
[0439]
Step 1
[0440]To a solution of compound 1B (650 mg, 2 mmol) in anhydrous THF (3 mL) was added MeMgBr (2.2 mL, 1.1 equiv., 2.2 mmol) at 0° C. The reaction was stirred at RT overnight before cooled to 0° C. A solution of NaBH4 in methanol (2 mL) was added and the reaction was further stirred at r.t. for 4 hr. Aq. HCl solution was added followed by stirring for another 1 hr. Aq. NaOH was added to make the pH around 9, and the reaction was extracted with DCM thoroughly. The organic layer was dried with Na2SO4 and concentrated to give the crude 2A which was used without further purification.
Step 2
[0441]To a solution of 2A (230 g, 1 mmol) in ethanol (3 mL) was added BrCN (0.4 mL, 5.0 M in CH3CN, 2.0 mmol) and the reaction was stirred at RT overnight. The mixture was diluted with aq. NaOH (1.0M, 10 mL) and extracted with DCM. The layers were separated. The combined organic layers were dried over Na2SO4, concentrated and chromatographed (2-5% of NH3-MeOH / DCM) to giv...
example 3
Preparative Example 3
[0443]
Step 1
[0444]
[0445]A solution of 5-bromo-tetralone (2.25 g, 10.0 mmol) in THF (100 mL) was cooled in an ice-H2O bath and a solution of allymagnesium bromide in THF (1.0M, 50 mL, 50 mmol) was added via a dropping funnel over a period of 25 min; stirring was continued at 0° C. for ½ h, then at RT for 16 h. NH4Cl (Sat., 100 mL) and EtOAc (200 mL) were added, the aqueous was extracted once more with EtOAc (100 mL). The combined organic was washed with brine (100 mL), dried over anhydrous MgSO4, and concentrated. The residue was purified by flash column chromatography on silica gel, eluting with EtOAc-Hexane (1:10) to obtain 3A as a yellow syrup. (2.28 g).
Step 2
[0446]
[0447]A solution of 3A (1.2 g, 4.50 mmol) in mixed solvent (15 mL CH2Cl2 and 7.5 mL MeOH) was cooled in a dry ice—acetone bath. Ozone was bubbled into it at a rate of 1.5 / min for 10 min and the solution turned blue. Oxygen was then blown into the mixture, followed by the addition of dimethyl sulfide...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Composition | aaaaa | aaaaa |
| Solubility (mass) | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com