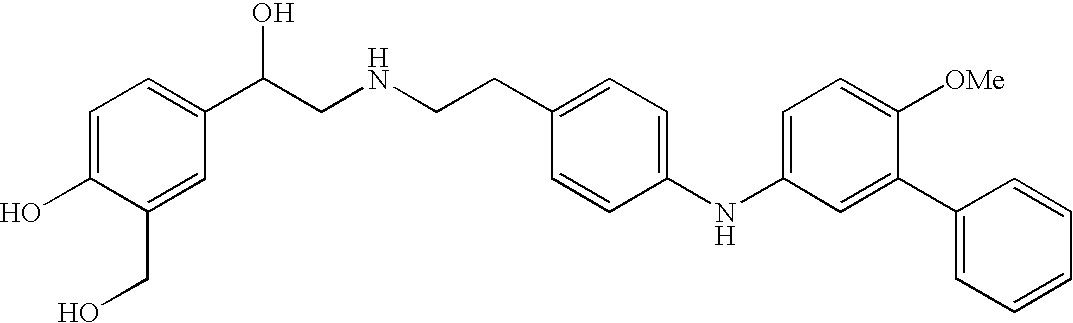
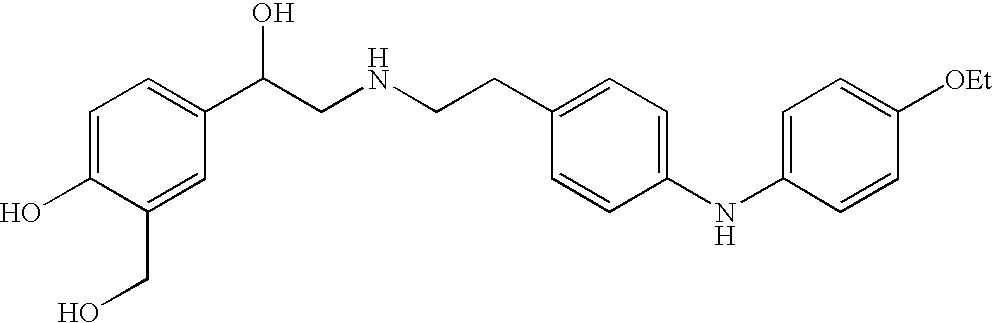
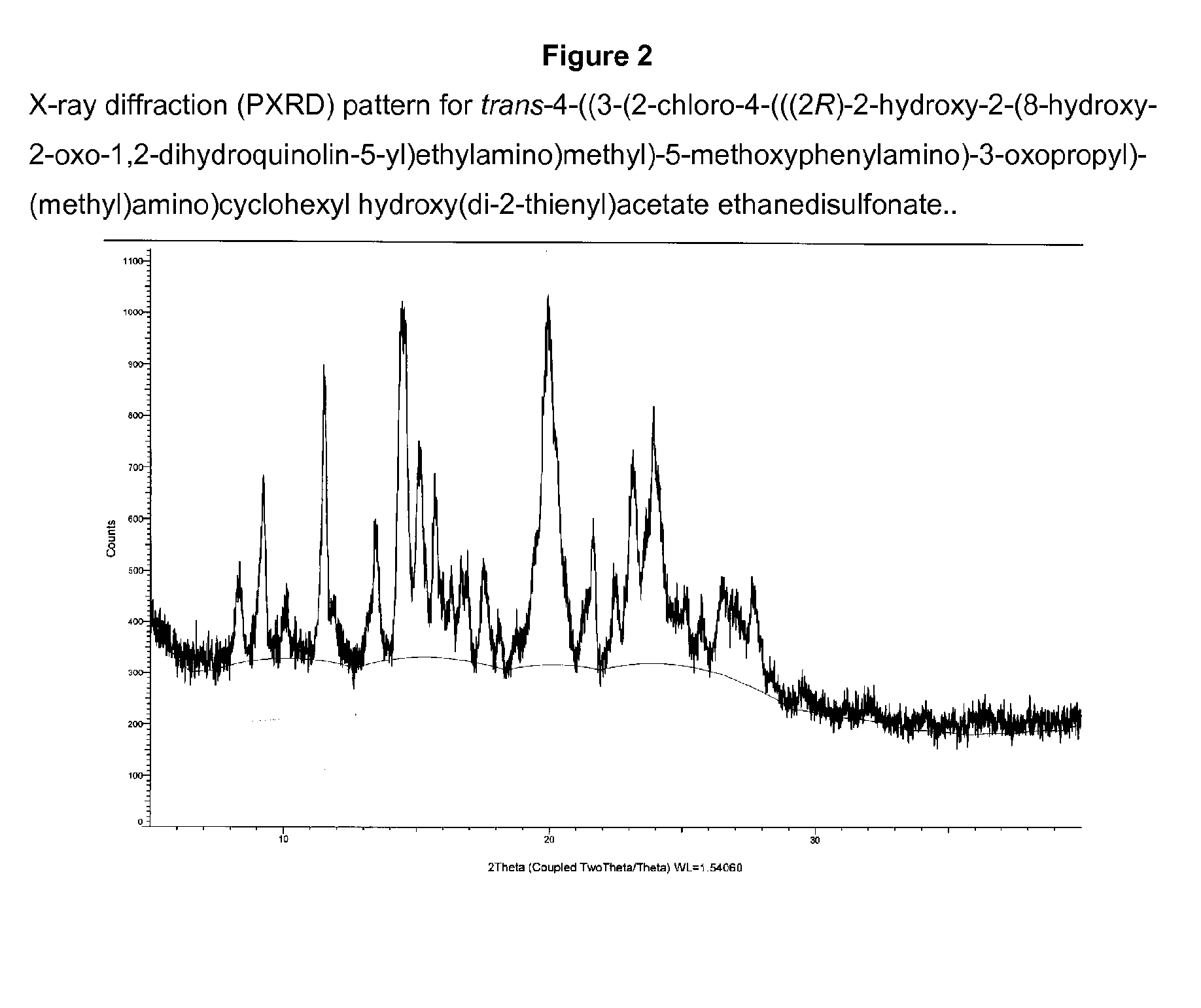
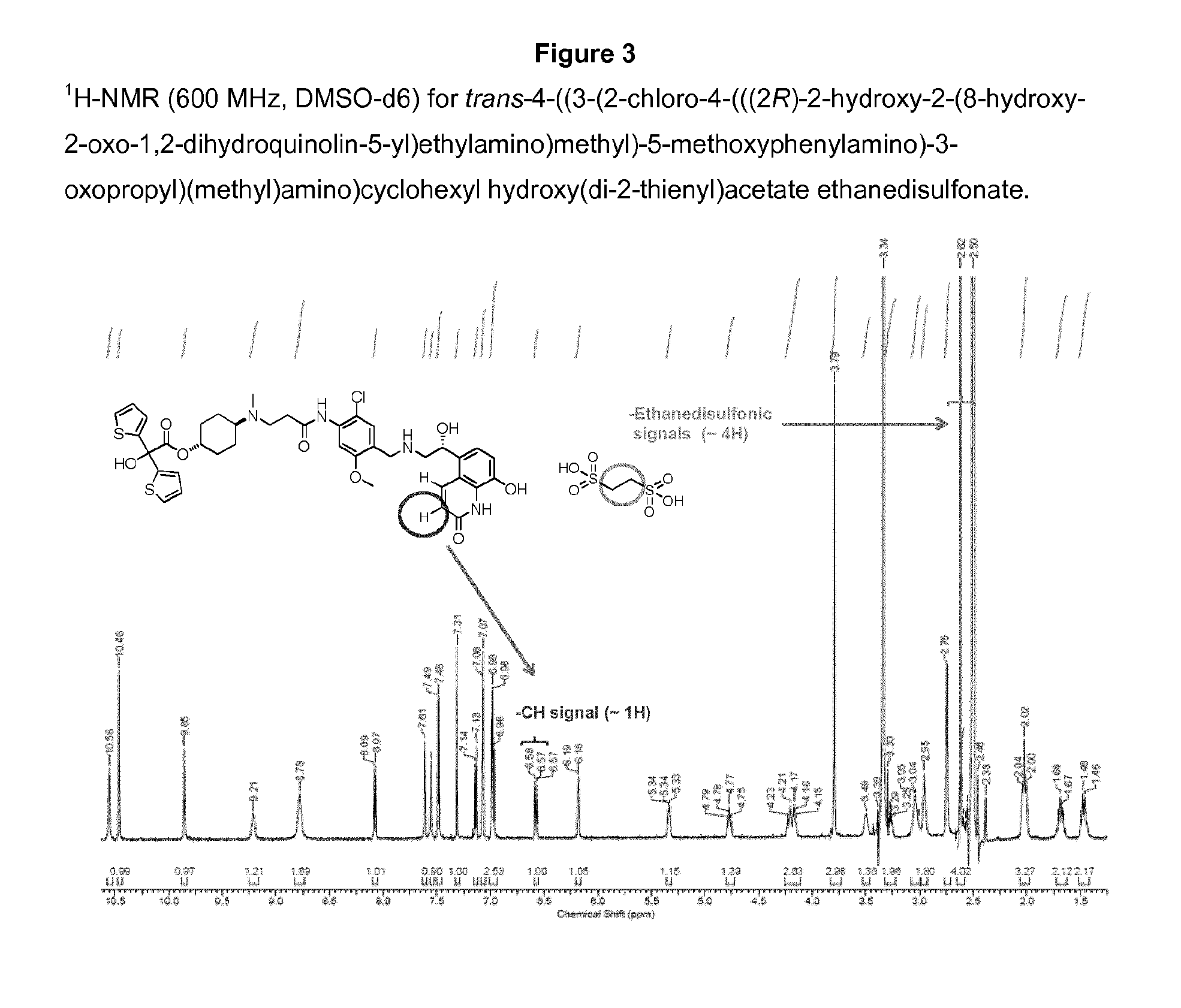
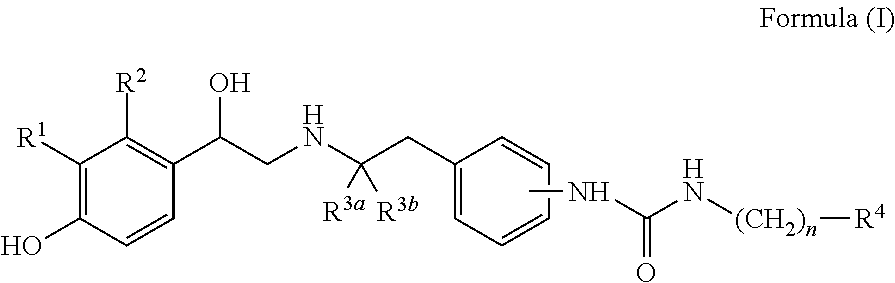
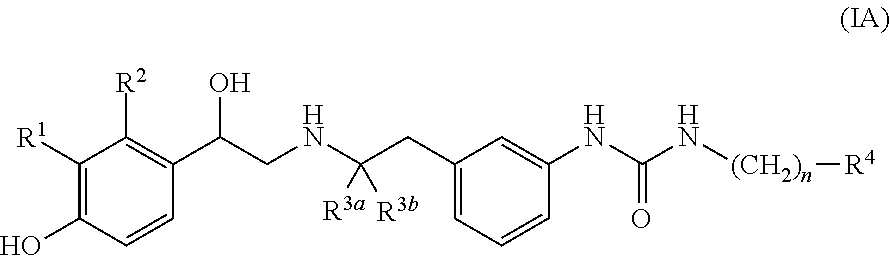
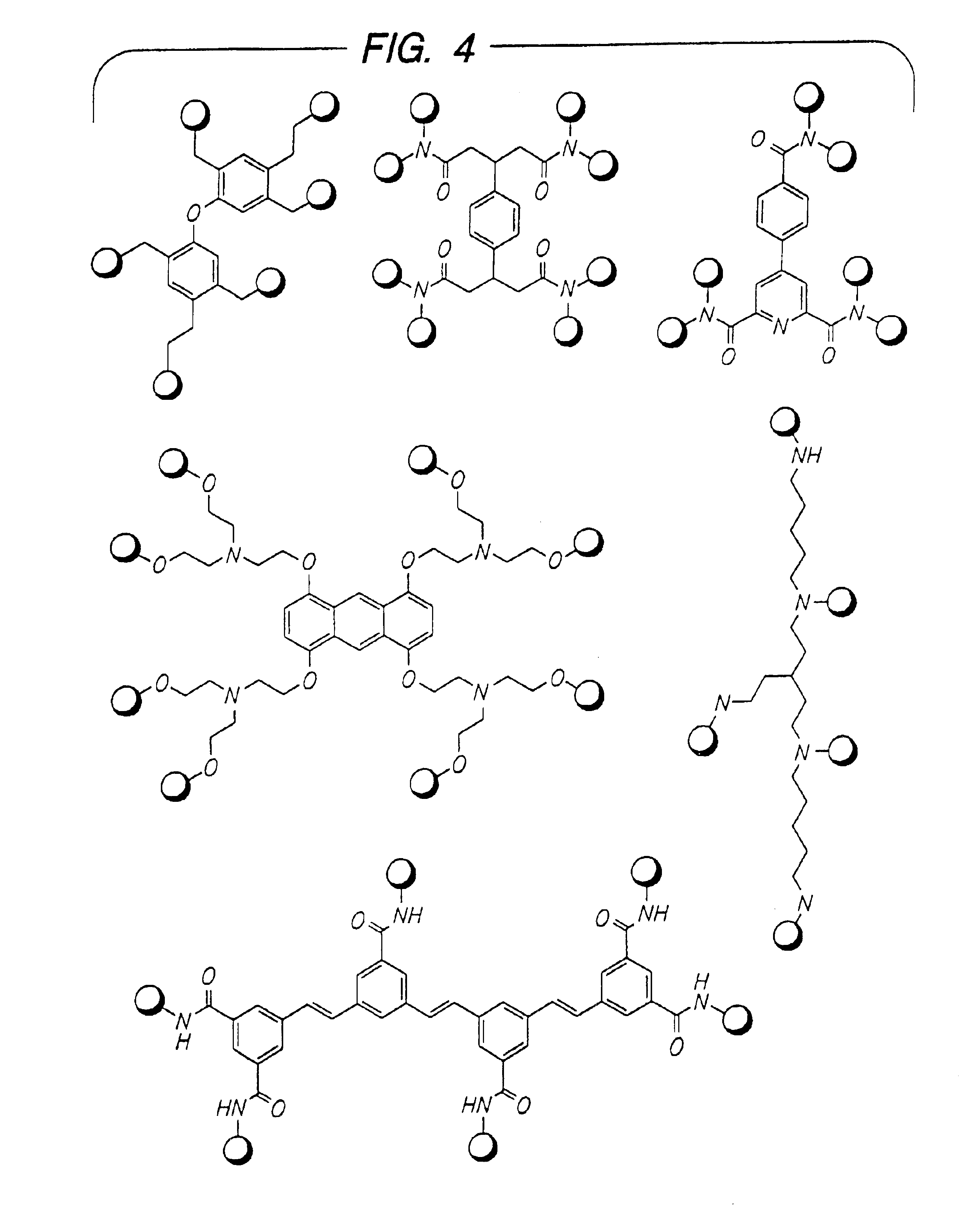Patents
Literature
82 results about "Α2 adrenergic receptor" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
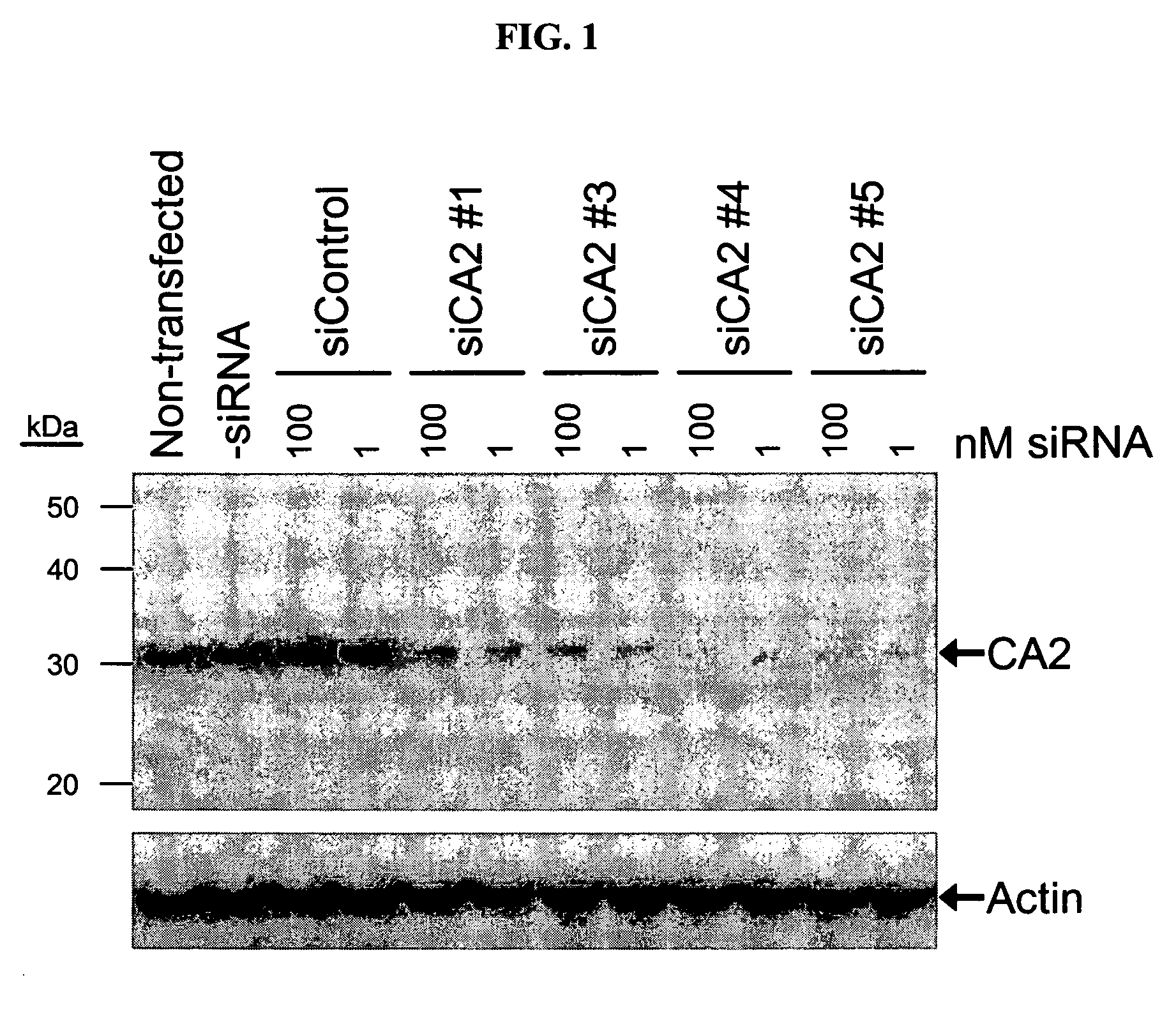
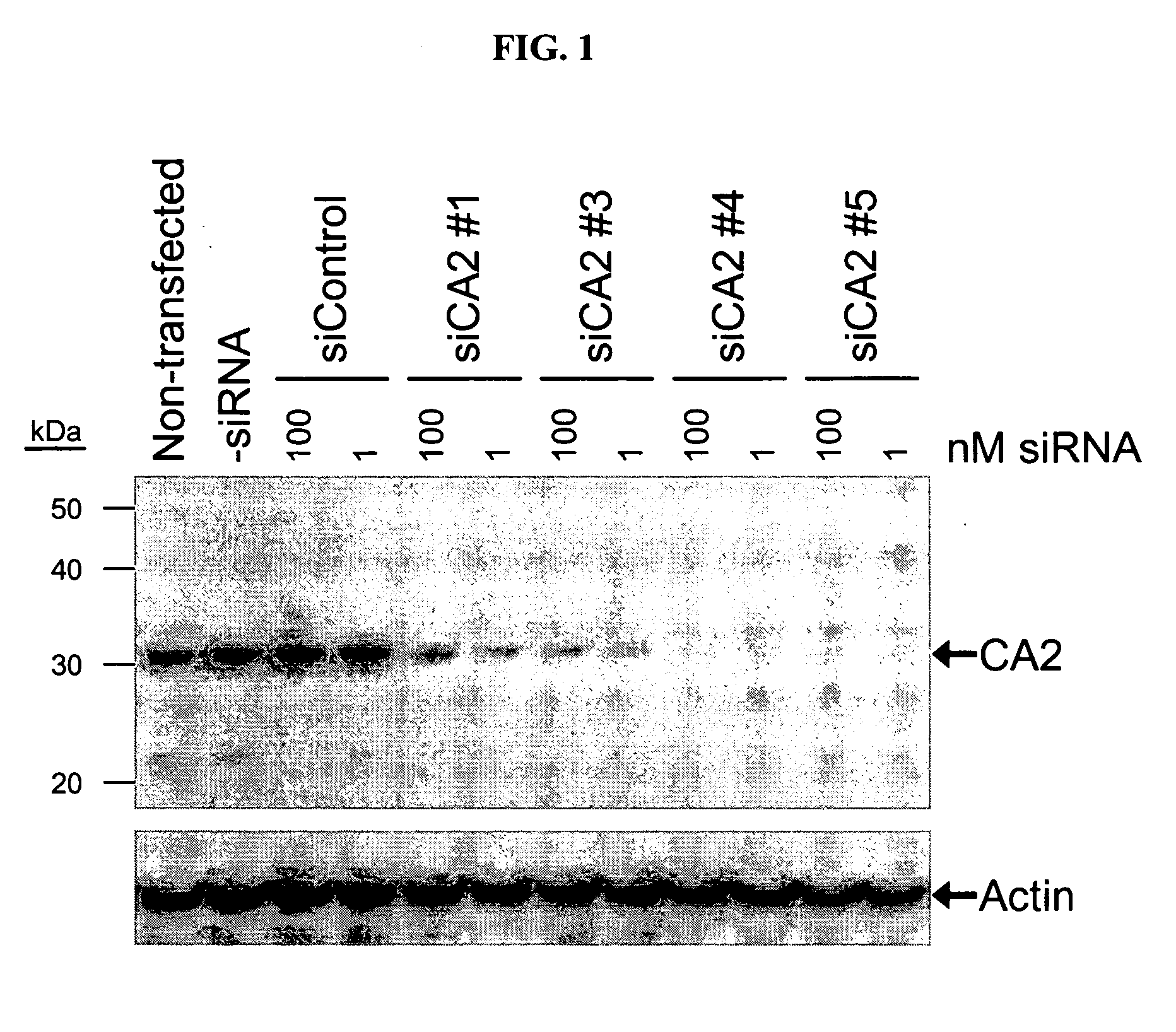
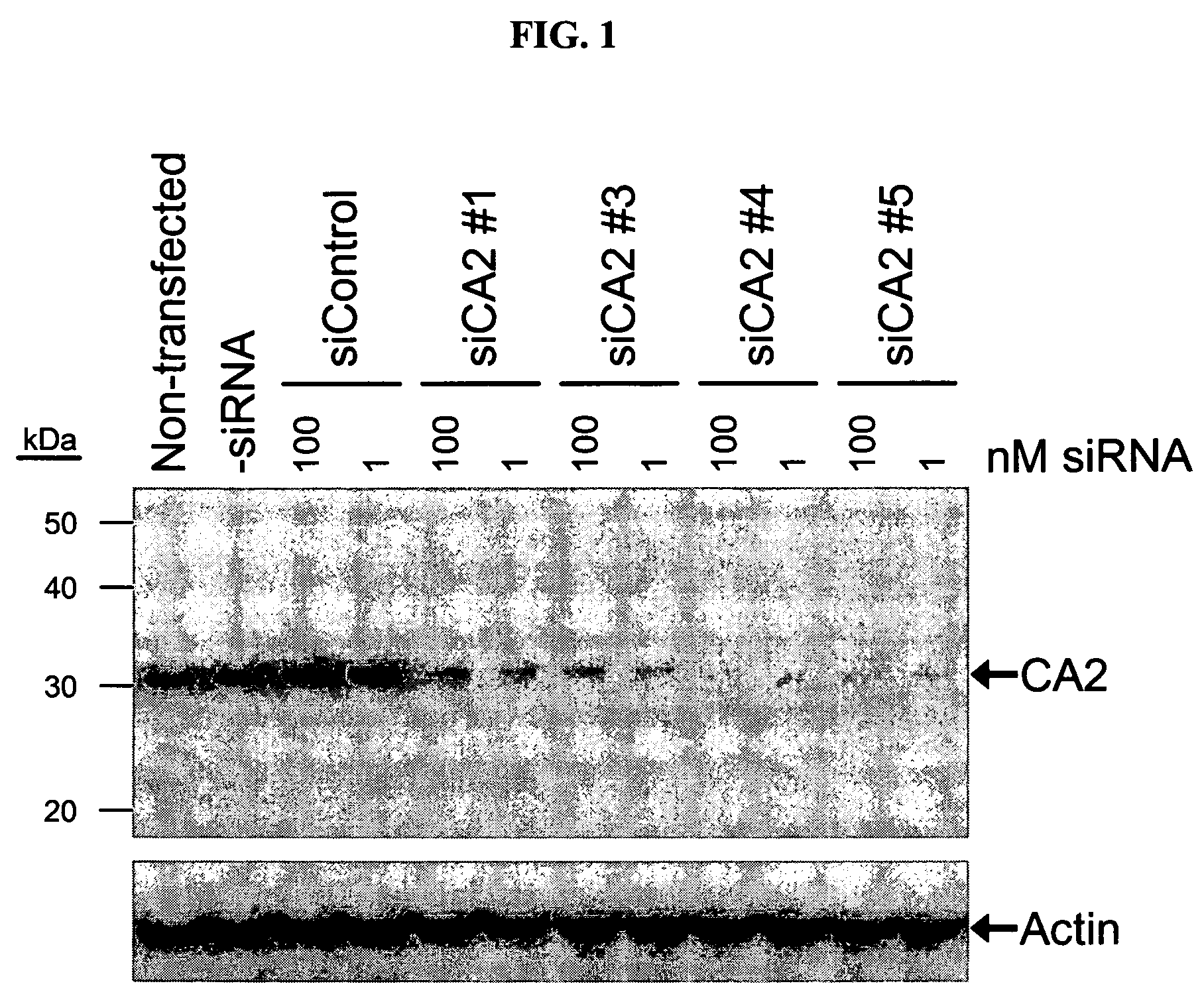
RNAi-mediated inhibition of ocular targets
InactiveUS20060172965A1Lower eye pressureOrganic active ingredientsSenses disorderATPaseOpen angle glaucoma
RNA interference is provided for inhibition of ocular hypertension target mRNA expression for lowering elevated intraocular pressure in patients with open-angle glaucoma or ocular hypertension. Ocular hypertension targets include carbonic anhydrase II, IV, and XII; β1- and β2 adrenergic receptors; acetylcholinesterase; Na+ / K+-ATPase; and Na—K-2Cl cotransporter. Ocular hypertension is treated by administering interfering RNAs of the present invention.
Owner:NOVARTIS AG
RNAi-mediated inhibition of ocular hypertension targets
InactiveUS20060172963A1Lower eye pressureOrganic active ingredientsSenses disorderIntra ocular pressureATPase
RNA interference is provided for inhibition of ocular hypertension target mRNA expression for lowering elevated intraocular pressure in patients with open-angle glaucoma or ocular hypertension. Ocular hypertension targets include carbonic anhydrase II, IV, and XII; β1- and β2 adrenergic receptors; acetylcholinesterase; Na+ / K+-ATPase; and Na—K-2Cl cotransporter. Ocular hypertension is treated by administering interfering RNAs of the present invention.
Owner:ARROWHEAD RES CORP +1
Method and Kit for Treating or Preventing Psoriasis
InactiveUS20110118267A1Treating and preventing psoriasisLittle side effectsBiocideInorganic non-active ingredientsGynecologyAdrenergic
Methods and kits for treating or preventing psoriasis or a symptom associated with psoriasis in a subject are described. The methods involve topical applications to the subject a therapeutically effective amount of an α2 adrenergic receptor agonist, such as brimonidine.
Owner:GALDERMA LAB LP
Methods and compositions for inhibition of beta2-adrenergic receptor degradation
The present invention generally relates to compositions and kits comprising a β2-AR agonist and a modulator of a β2-AR regulator gene, where the modulator of the β2-AR regulator gene inhibits the internalization and / or degradation of the β2-ad-renergic receptor (β2-AR). More specifically, the present invention relates to the use of an agonist of β2-adrenergic receptor (β2-AR) and an agent which inhibits agonist induced β2-adrenergic receptor (β2-AR) internalization and / or degradation in method for the treatment of a respiratory disorder in a subject.
Owner:PRESIDENT & FELLOWS OF HARVARD COLLEGE
RNAi-mediated inhibition of ocular targets
RNA interference is provided for inhibition of ocular hypertension target mRNA expression for lowering elevated intraocular pressure in patients with open-angle glaucoma or ocular hypertension. Ocular hypertension targets include carbonic anhydrase II, IV, and XII; β1- and β2 adrenergic receptors; acetylcholinesterase; Na+ / K+-ATPase; and Na—K-2Cl cotransporter. Ocular hypertension is treated by administering interfering RNAs of the present invention.
Owner:NOVARTIS AG
Compounds, Formulations and Methods for Reducing Skin Wrinkles, Creasing and Sagging
InactiveUS20090060852A1Reducing skin wrinkleReduce creasesBiocideCosmetic preparationsWrinkle skinΑ2 adrenergic receptor
Methods, compounds, and topical formulations for reduction of skin sagging, creasing and / or wrinkling are disclosed. The methods comprise topically applying a composition comprising an α2 adrenergic receptor agonist. Amelioration of skin sagging, creasing and / or wrinkling begins within minutes after topical application of a disclosed composition. A single application can significantly reduce skin sagging, creasing and / or wrinkling for at least about 8 hours.
Owner:GALDERMA LAB LP
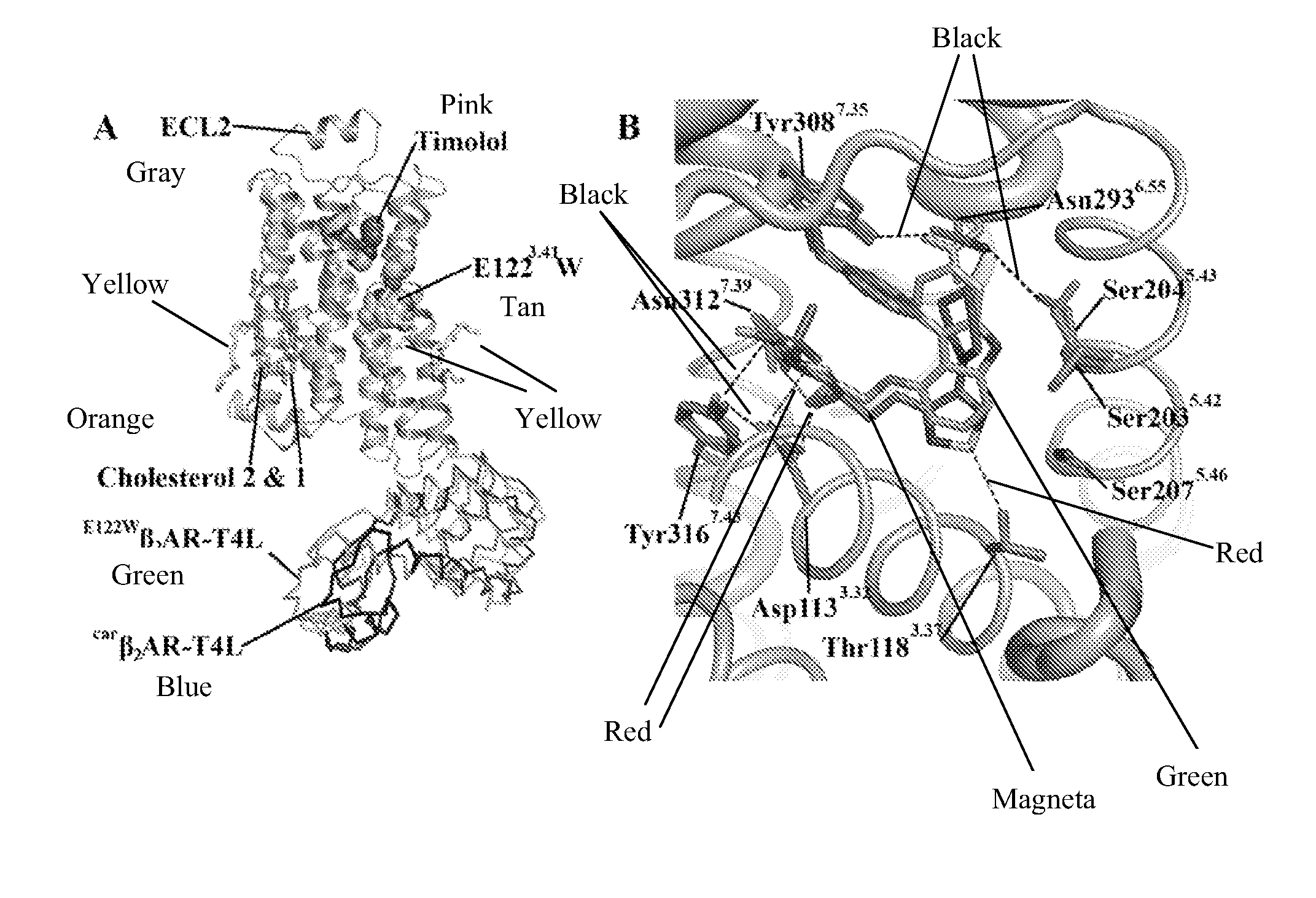
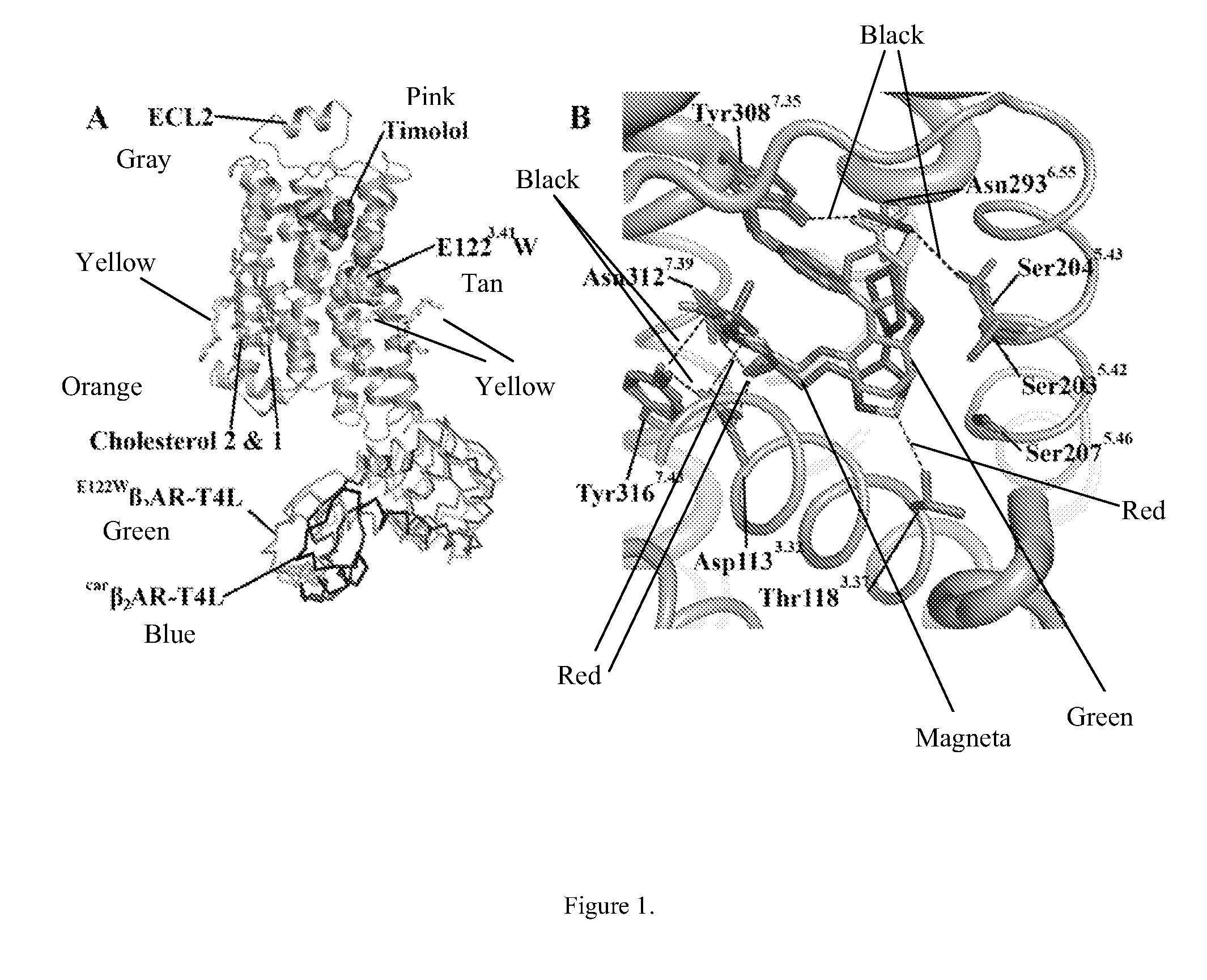
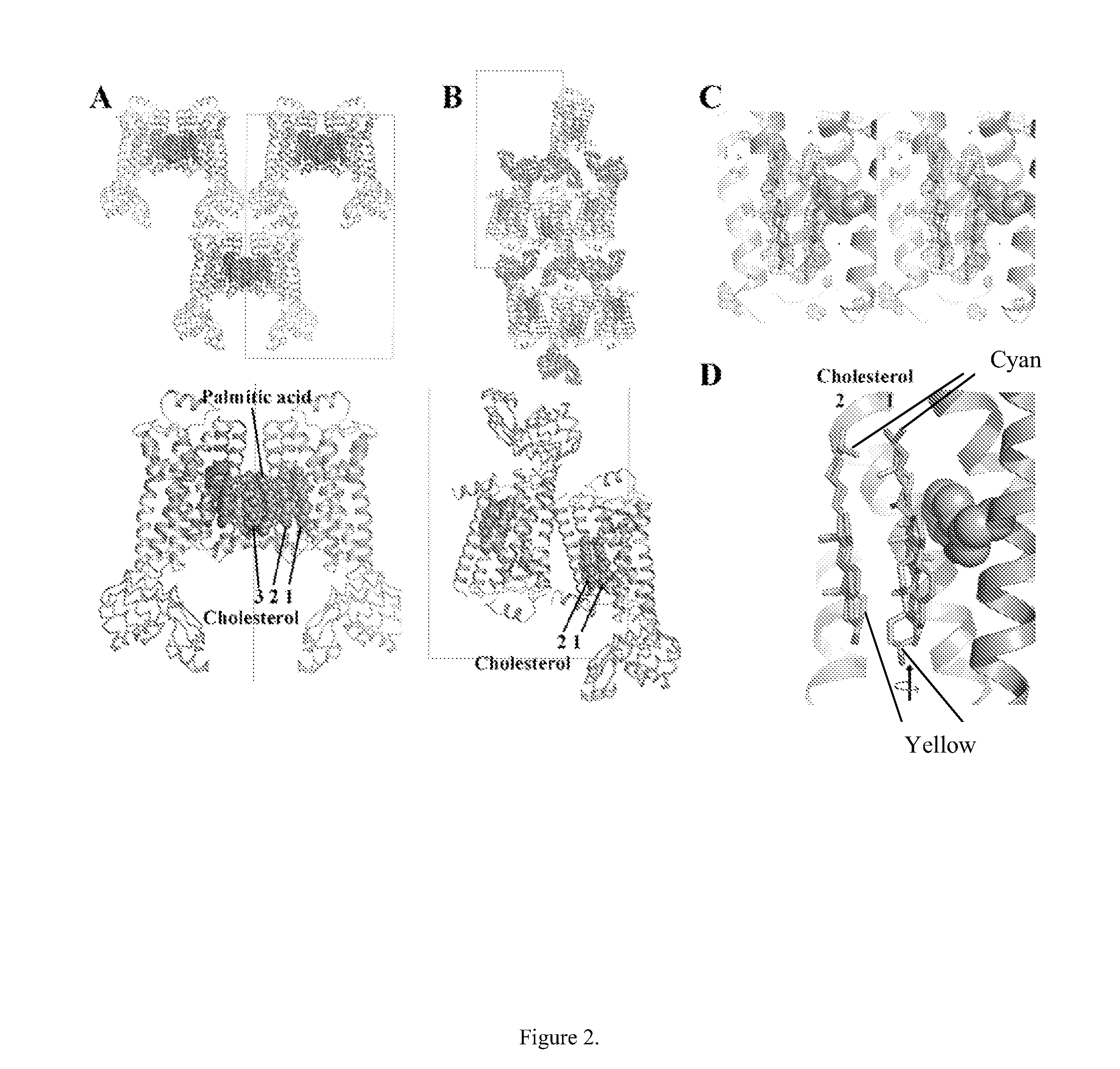
Cholesterol consensus motif of membrane proteins
InactiveUS20110130543A1Improve thermal stabilityIncrease constraintsCell receptors/surface-antigens/surface-determinantsLibrary screeningAdrenergicG protein-coupled receptor
The invention provides the structure of a human β2-adrenergic receptor, a cholesterol consensus motif, and methods of identifying modulators of G-protein coupled receptors (GPCRs). Methods of using the modulators of the receptor, GPCRs, and the cholesterol consensus motif are also provided.
Owner:THE SCRIPPS RES INST
Aryl aniline beta2 adrenergic receptor agonists
The invention provides novel β2 adrenergic receptor agonist compounds. The invention also provides pharmaceutical compositions comprising such compounds, methods of using such compounds to treat diseases associated with β2 adrenergic receptor activity, and processes and intermediates useful for preparing such compounds.
Owner:THERAVANCE BIOPHARMA R&D IP LLC
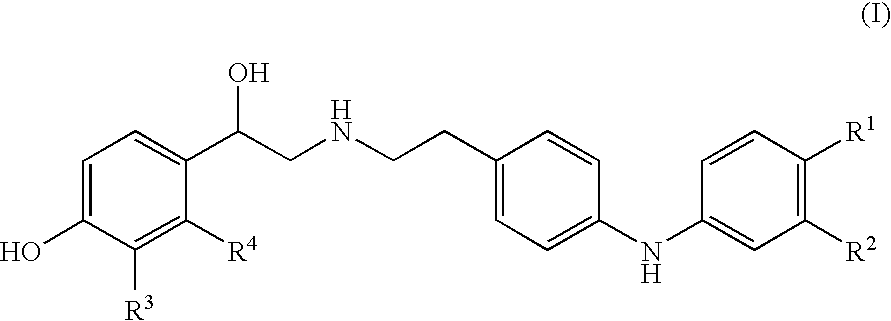
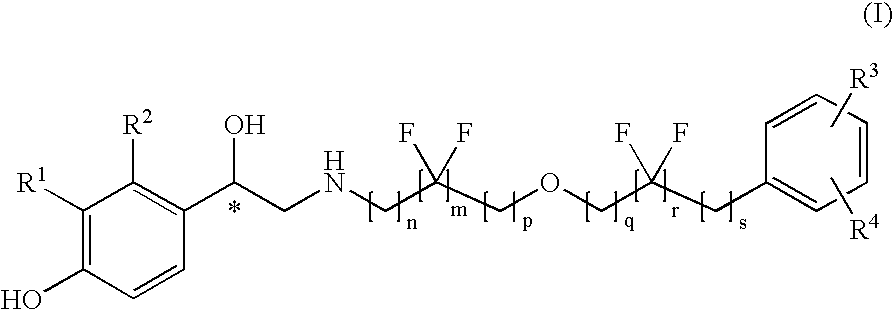
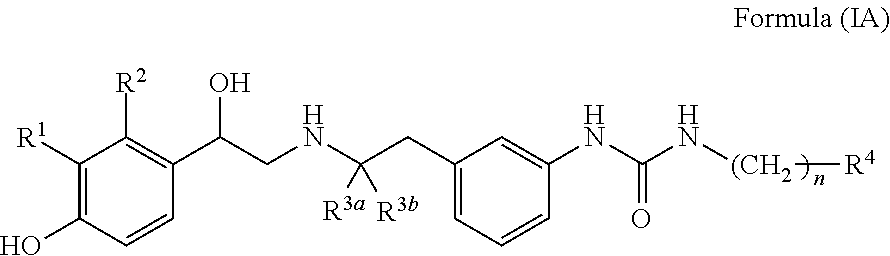
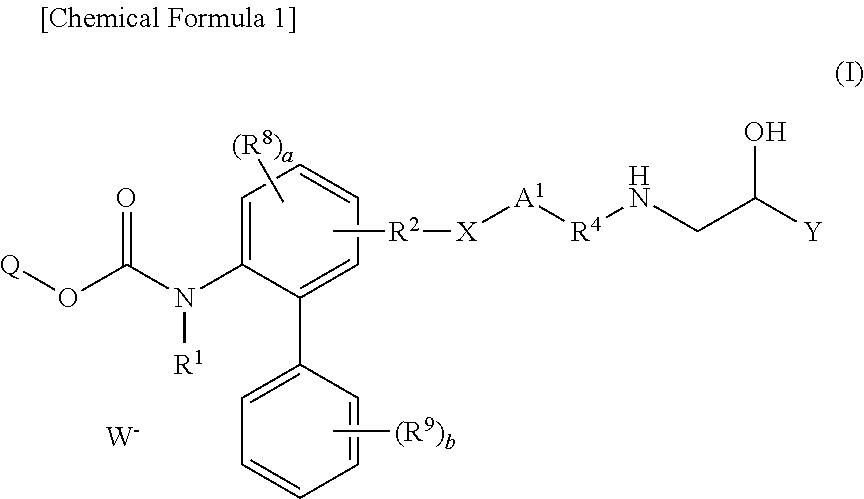
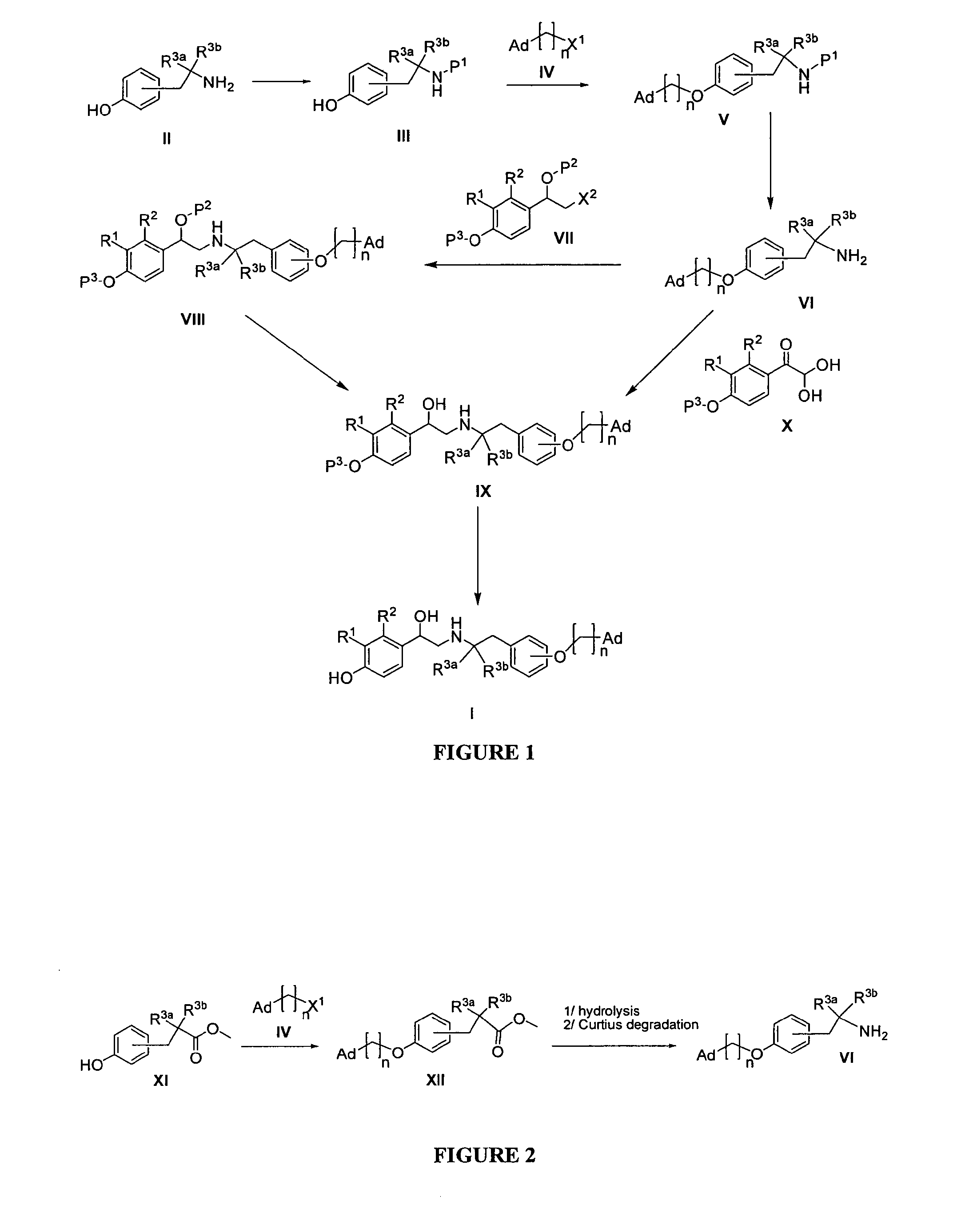
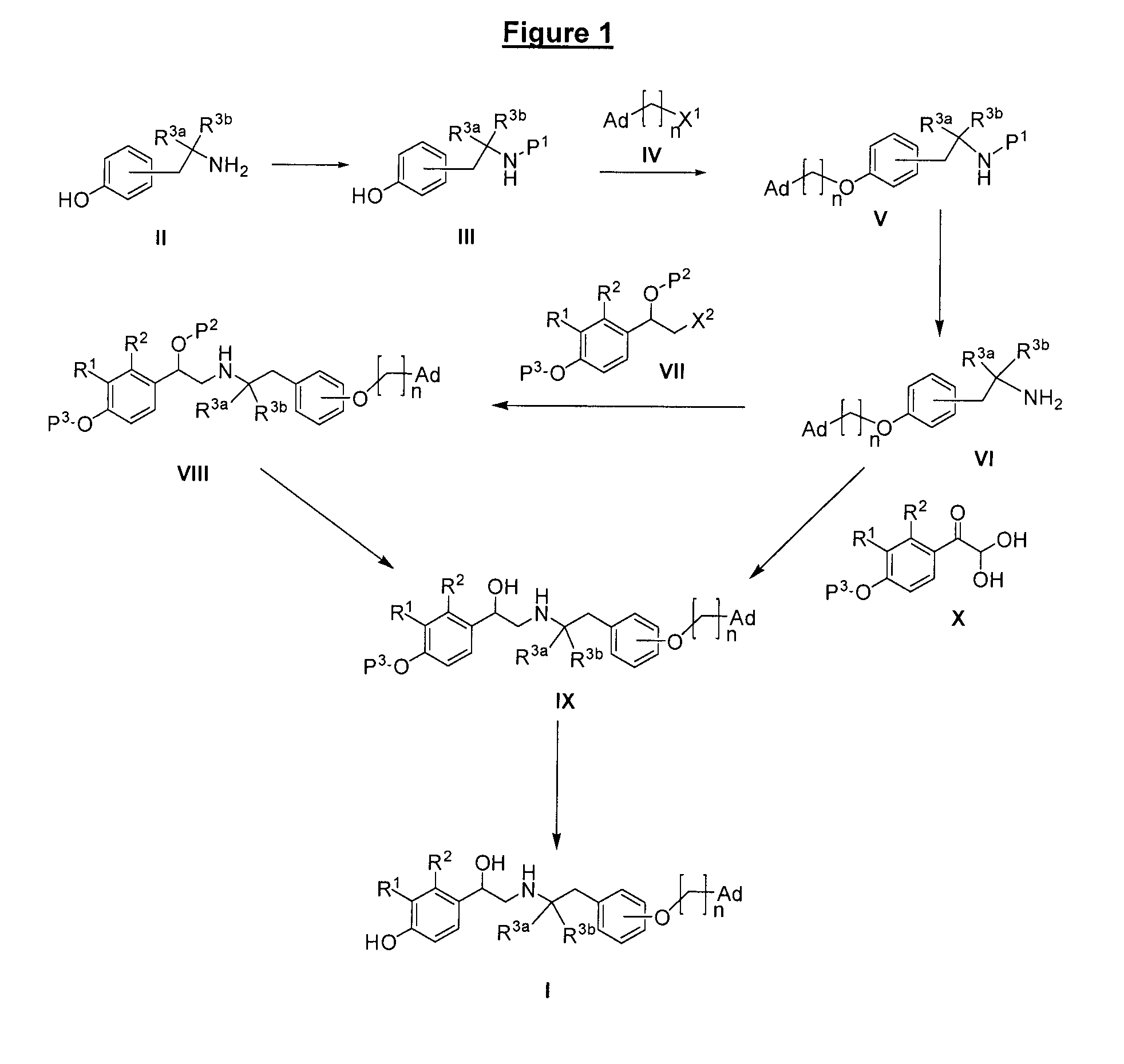
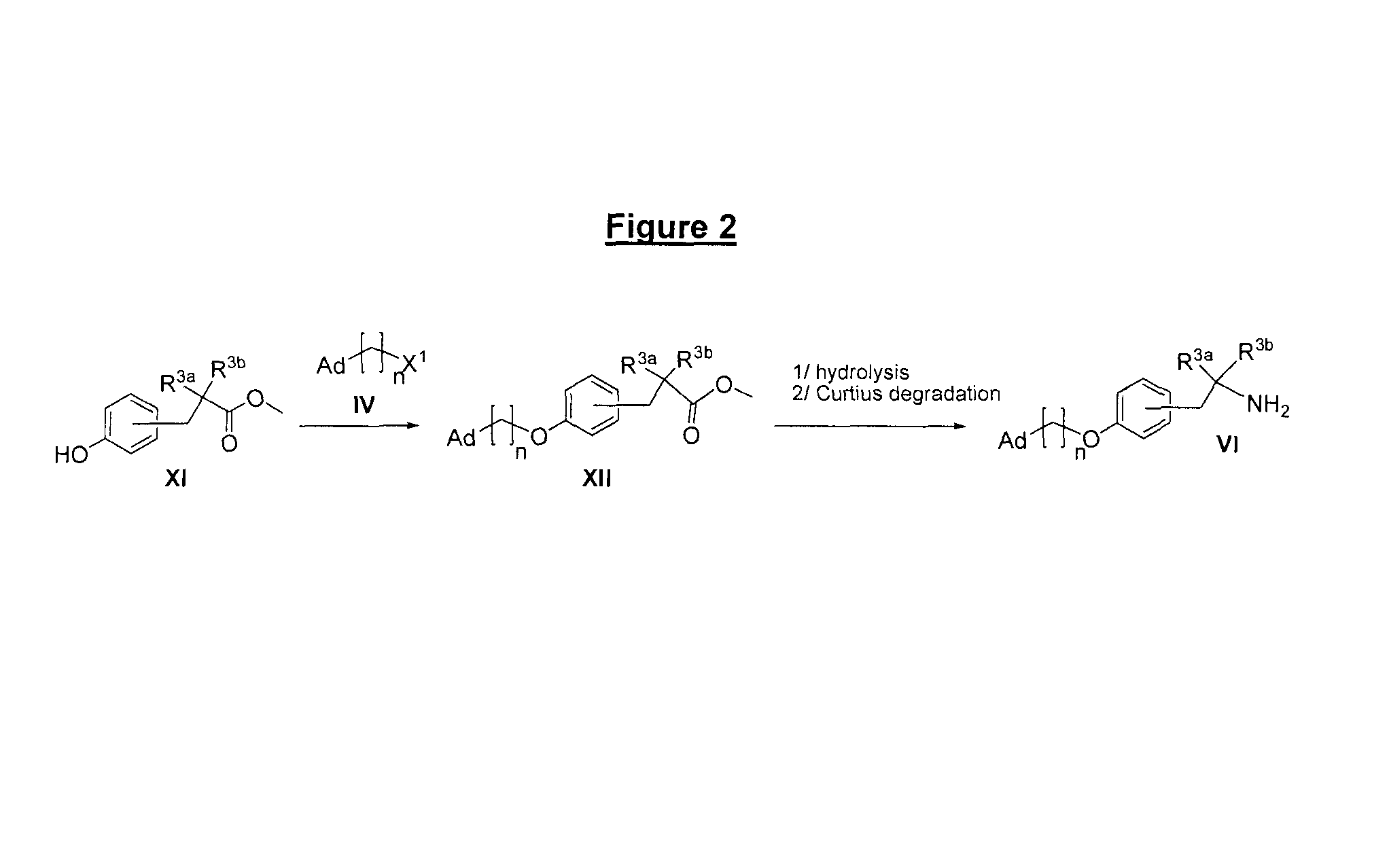
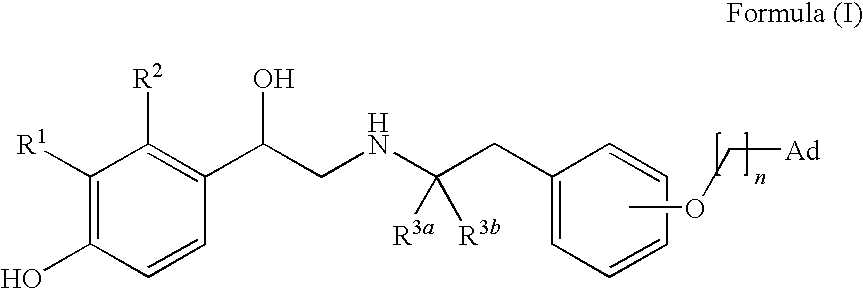
Derivatives of 4-(2-amino-1-hydroxyethyl)phenol as agonists of the β2 adrenergic receptor
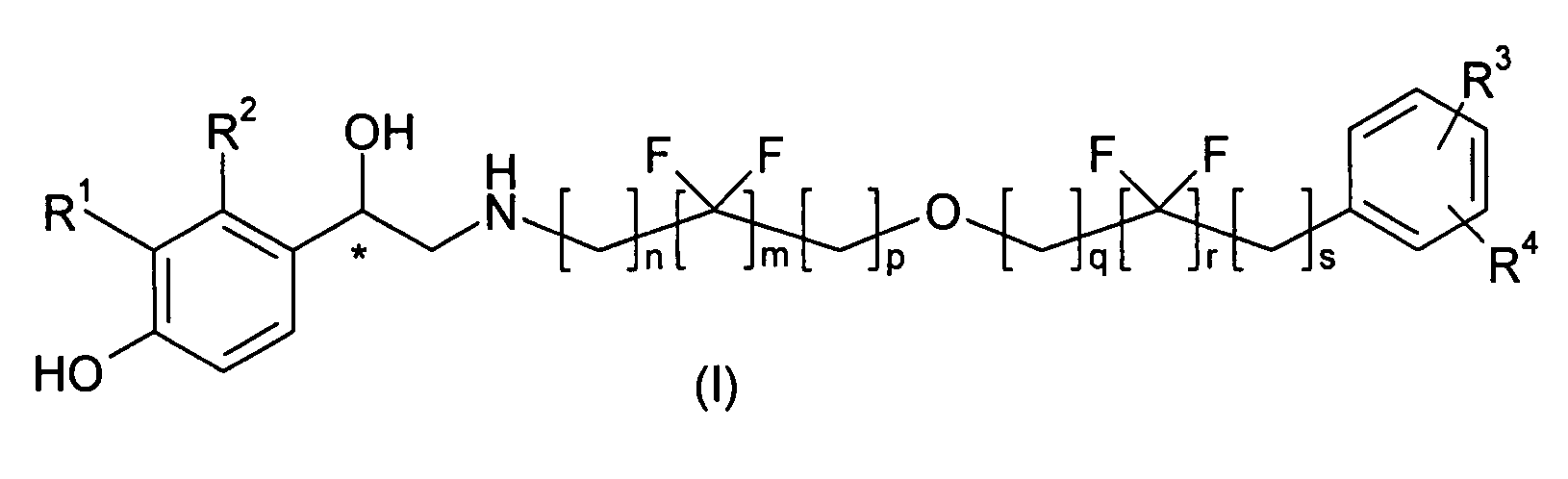
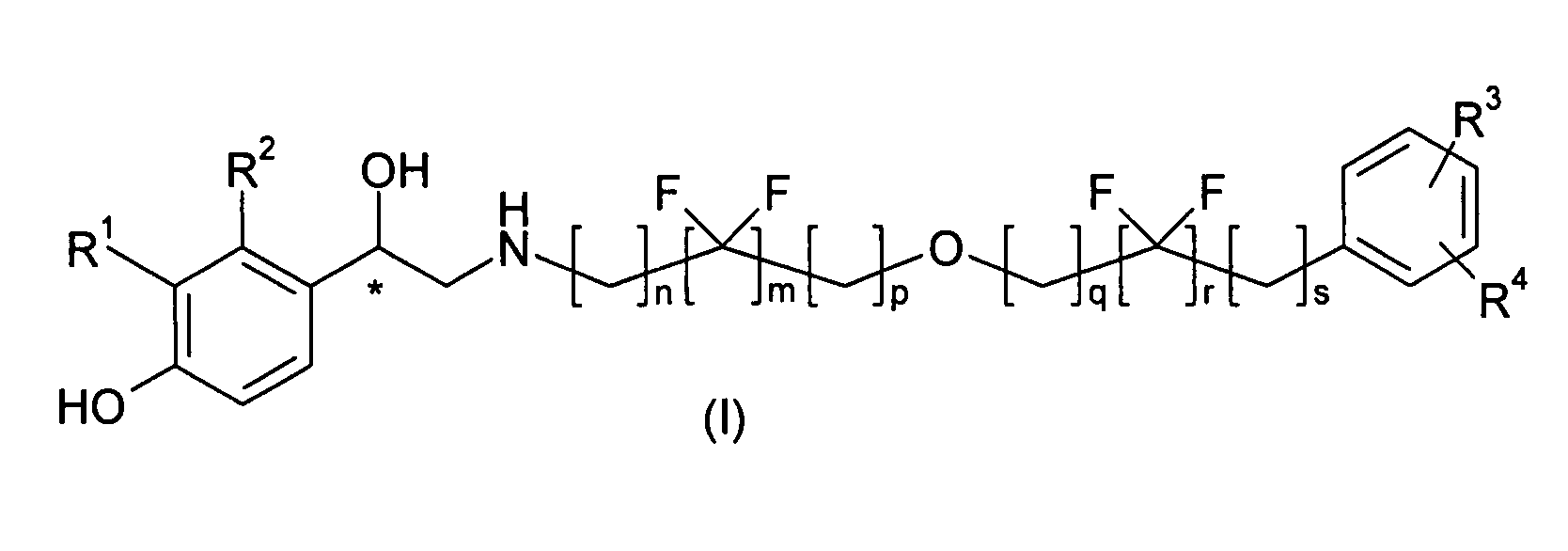
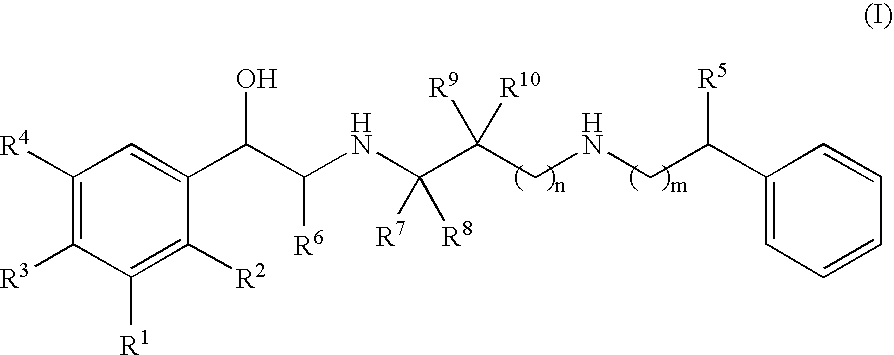
A compound of formula (I) or a pharmaceutically-acceptable salt, solvate or stereoisomer thereof wherein R1 is a group chosen from —CH2OH and —NHC(O)H; R2 is a hydrogen atom or R1 together with R2 form the group —NH—C(O)—CH═CH—, wherein the nitrogen atom is bound to the carbon atom in the phenyl ring holding R1 and the carbon atom is bound to the carbon atom in the phenyl ring holding R2; R3 is chosen from a hydrogen atom, a halogen atom and groups chosen from —SO—R5, —SO2—R5, —NH—CO—NH2, —CO—NH2, hydantoino, C1-4alkyl, C1-4alkoxy and —SO2NR5R6; R4 is chosen from a hydrogen atom, a halogen atom and a C1-4 alkyl group; R5 is chosen from a C1-4 alkyl group and a C3-8 cycloalkyl group; R6 is independently chosen from a hydrogen atom and a C1-4 alkyl group; n, p and q are independently 0, 1, 2, 3 or 4; m and s are independently 0, 1, 2 or 3; and r is 0, 1 or 2 with the provisos that at least one of m and r is not 0, the sum n+m+p+q+r+s is 7, 8, 9, 10, 11, 12 or 13, and the sum q+r+s is 2, 3, 4, 5 or 6.
Owner:ALMIRALL
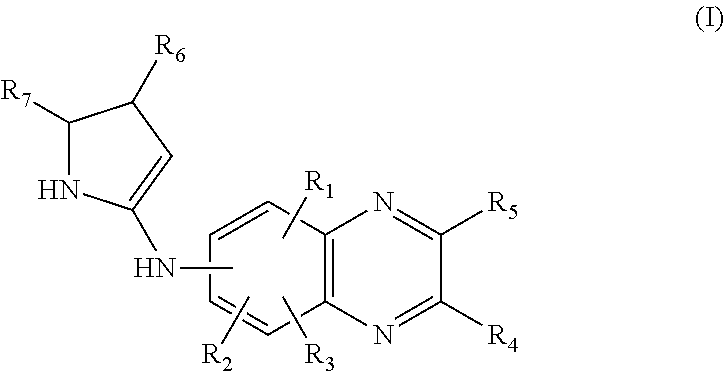
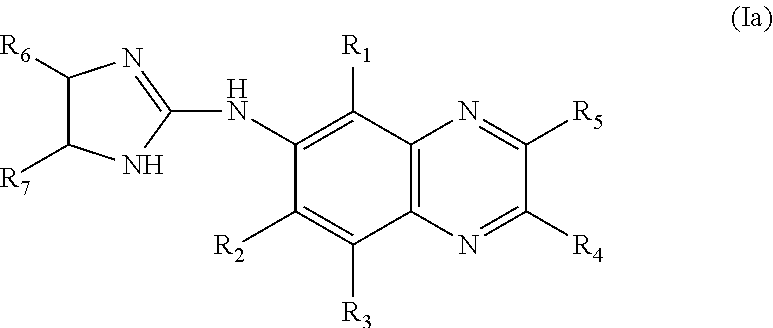
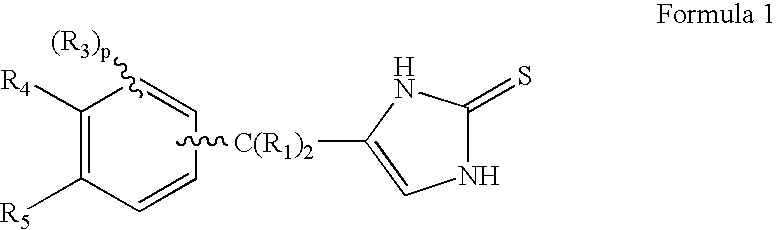
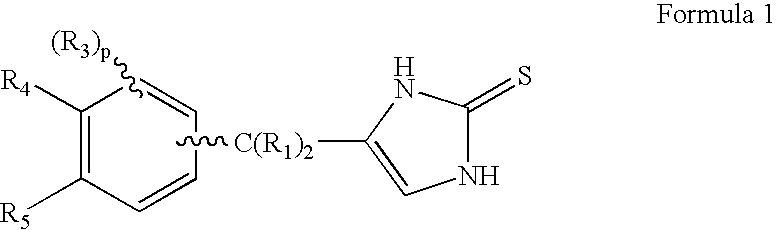
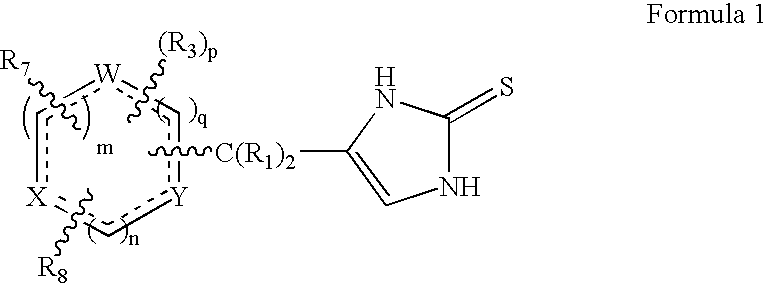
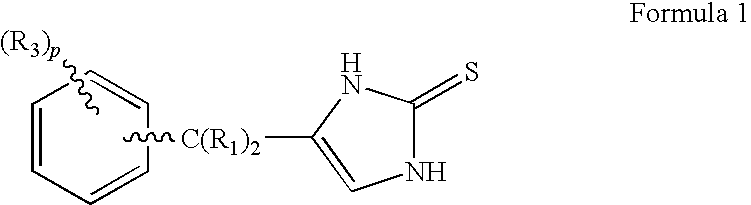
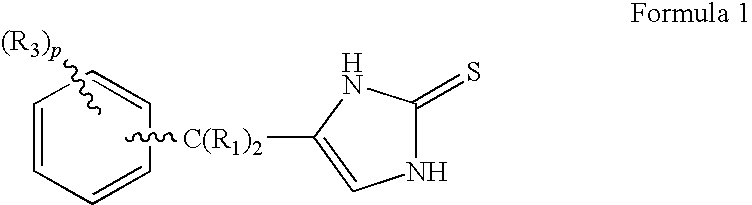
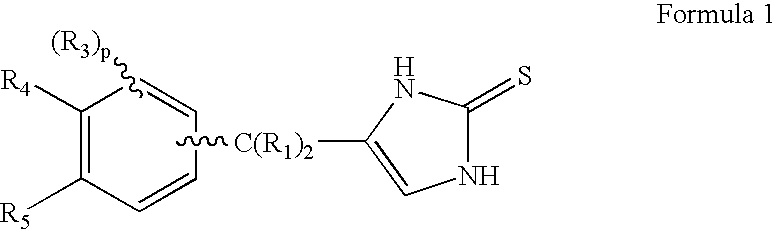
4-(Condensed cyclicmethyl)-imidazole-2-thiones acting as alpha2 adrenergic agonists
InactiveUS20060069144A1Minimal cardiovascularMinimal sedatory activityBiocideSenses disorderDiseaseSide effect
Compounds of Formula 1 where the variables have the meaning defined in the specification are agonists of alpha2 adrenergic receptors. Several compounds of the disclosure are specific or selective to alpha2B and / or alpha2C adrenergic receptors in preference over alpha2A adrenergic receptors. Additionally some of the claimed compounds have no or only minimal cardivascular and / or sedatory activity. The compounds of Formula 1 are useful as medicaments in mammals, including humans, for treatment of diseases and or alleviations of conditions which are responsive to treatment by agonists of alpha2 adrenergic receptors. Compounds of Formula 1 which have no significant cardiovascular and / or sedatory activity are useful for treating pain and other conditions with minimal side effects.
Owner:ALLERGAN INC
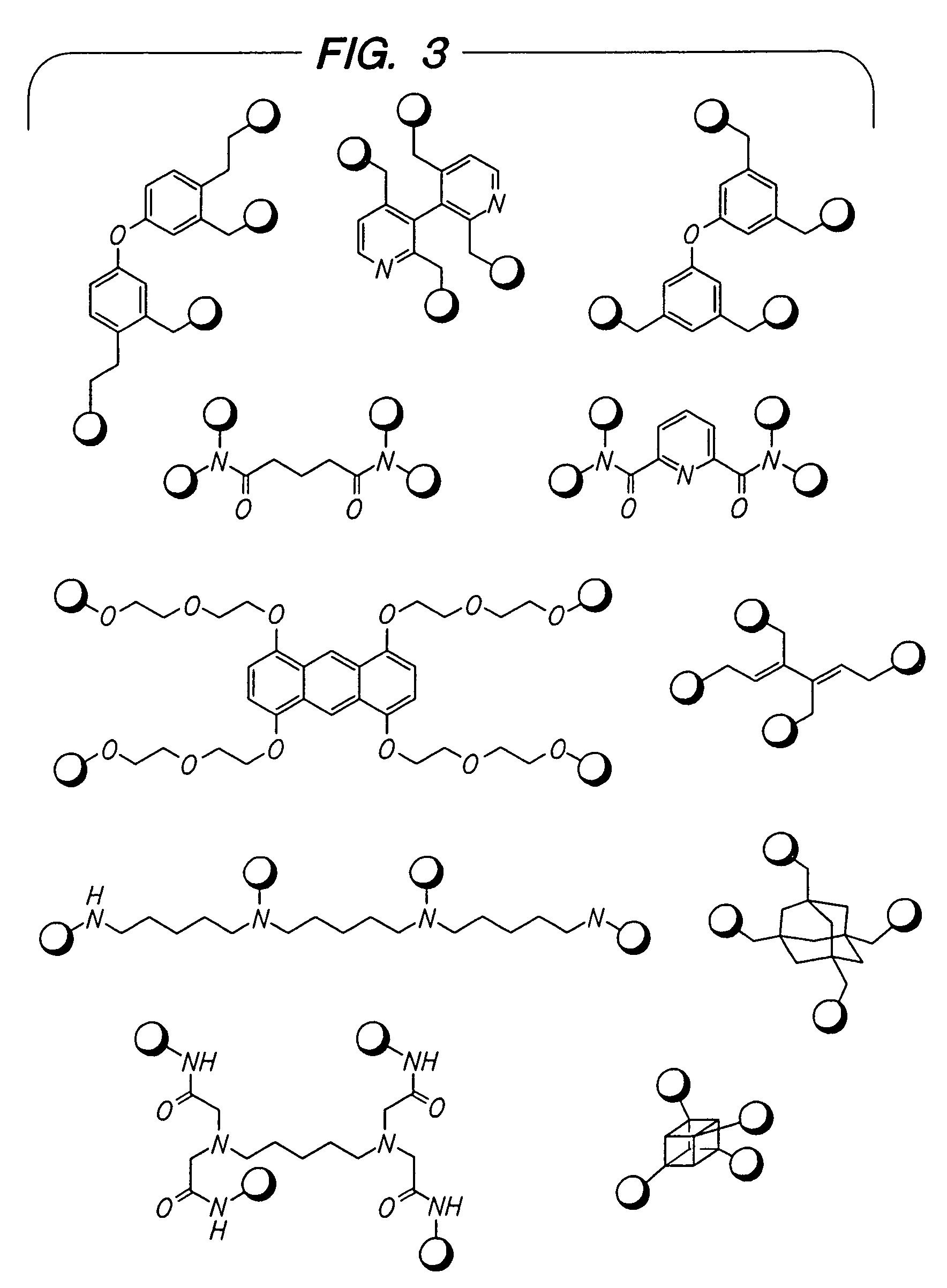
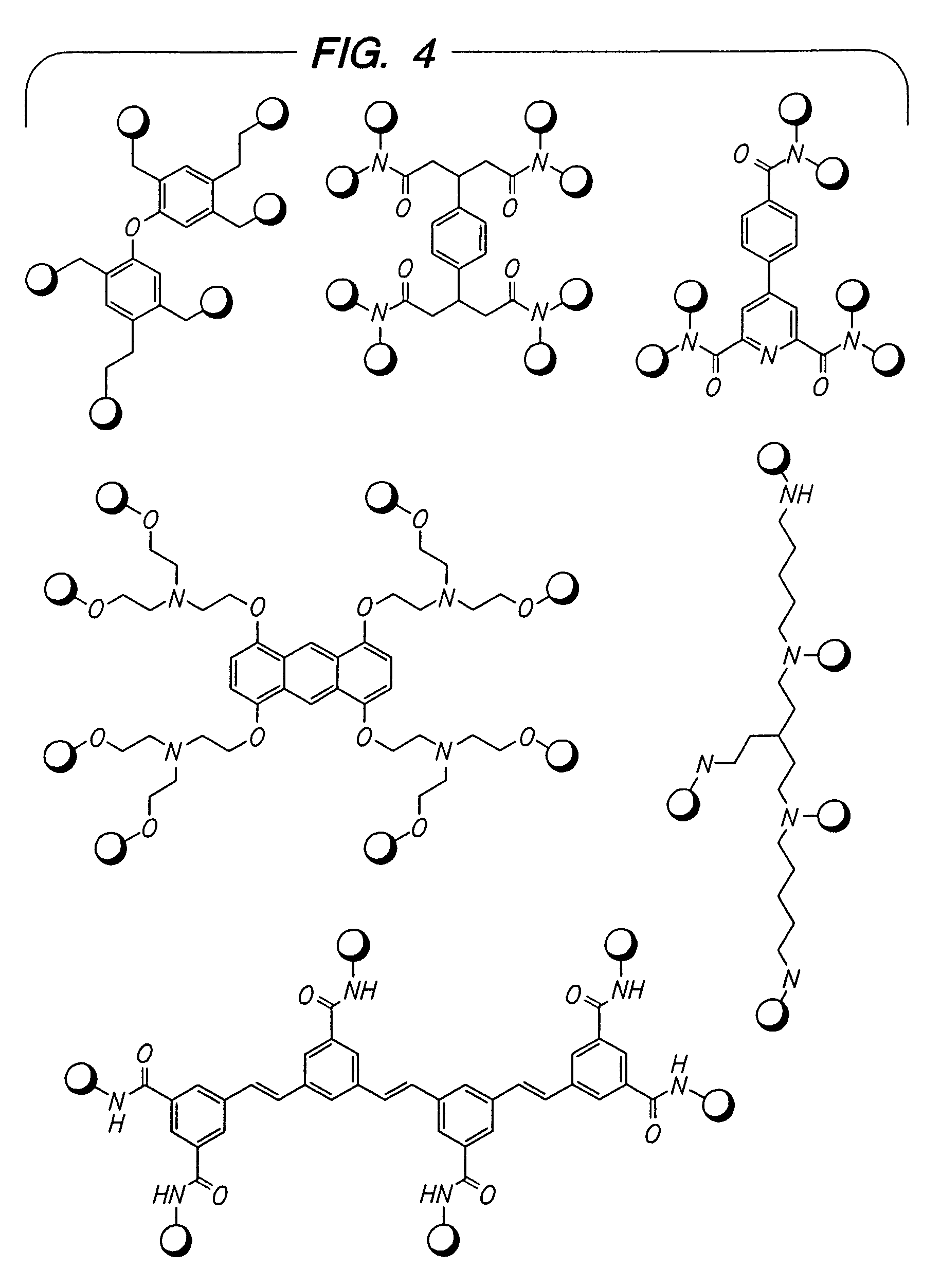
Beta2-adrenergic receptor agonists
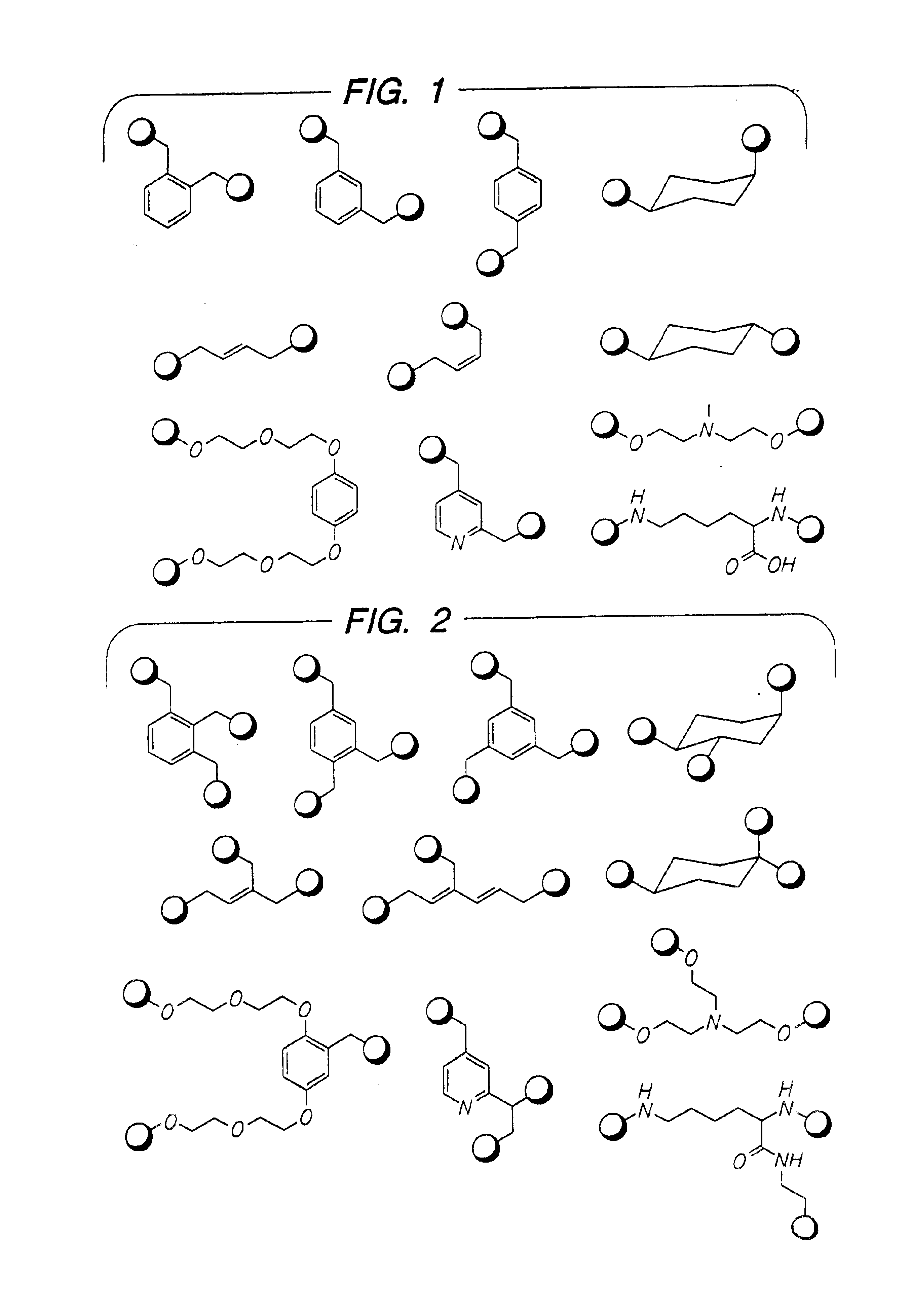
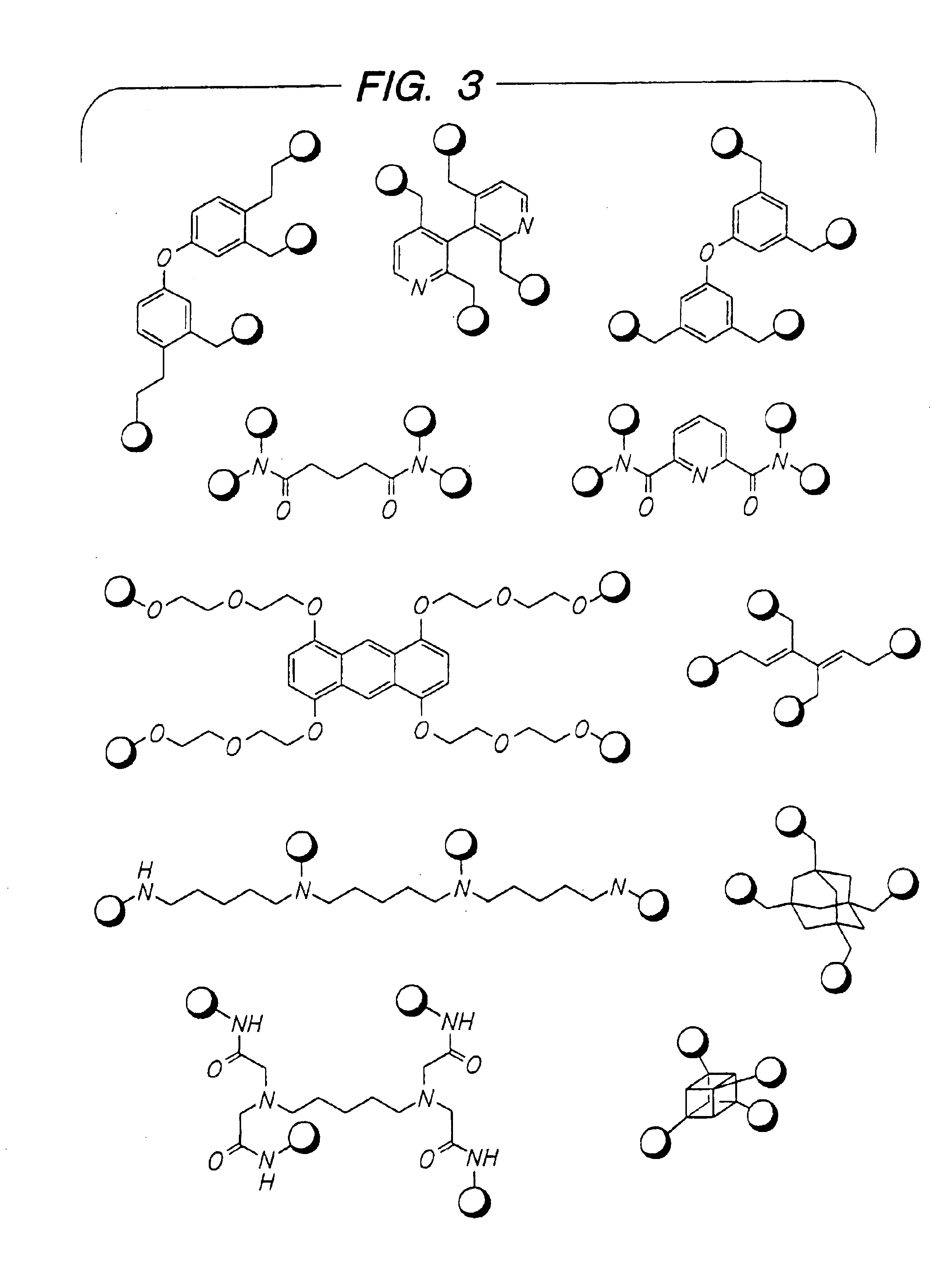
Disclosed are multibinding compounds which are β2-adrenergic receptor agonists and are useful in the treatment and prevention of respiratory diseases such as asthma, bronchitis. They are also useful in the treatment of nervous system injury and premature labor.
Owner:THERAVANCE BIOPHARMA R&D IP LLC
Salts of 2-amino-1-hydroxyethyl-8-hydroxyquinolin-2(1H)-one derivatives having both β2 adrenergic receptor agonist and m3 muscarinic receptor antagonist activities
The present invention is directed to crystalline addition salts of (i) 8-hydroxyquinolin-2(1H)-one derivatives and (ii) a hydroxycarboxylic acid, a sulfonic acid or a sulfimide, or a pharmaceutically acceptable solvates thereof.
Owner:ALMIRALL
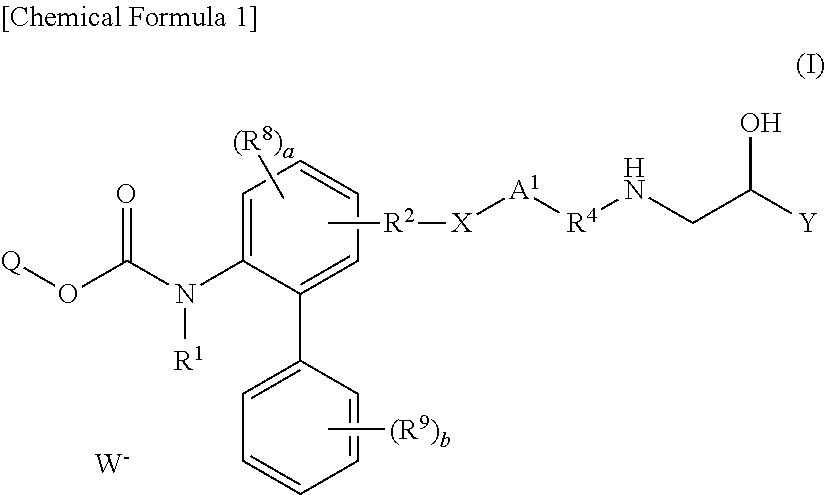
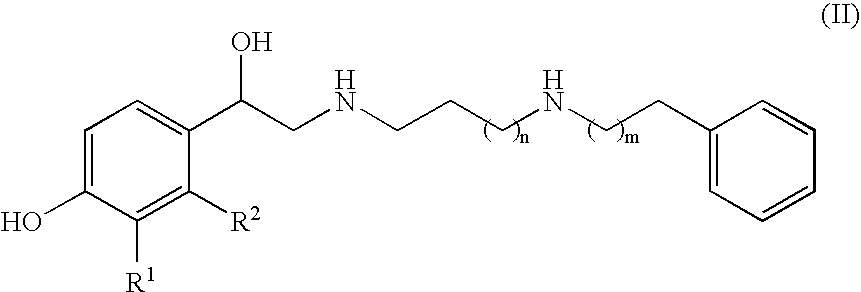
Derivatives of 4-(2-amino-1-hydroxyethyl) phenol as agonists of the beta2 adrenergic receptor
The present disclosure relates to 4-(2-amino-1-hydroxyethyl)phenol derivatives of formula (I) as well as pharmaceutical compositions comprising them, and their use in therapy as agonists of the β2 adrenergic receptor.
Owner:ALMIRALL
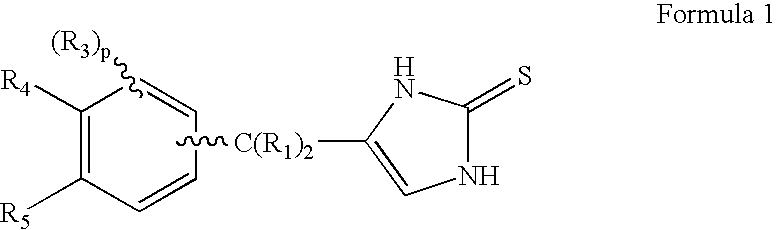
4-(Heteroaryl-methyl and substituted heteroaryl-methyl)-imidazole-2-thiones acting as alpha2 adrenergic agonists
Compounds of Formula 1where the variables have the meaning defined in the specification are agonists of alpha2 adrenergic receptors. Several compounds of the disclosure are specific or selective to alpha2B and / or alpha2C adrenergic receptors in preference over alpha2A adrenergic receptors. Additionally some of the claimed compounds have no or only minimal cardivascular and / or sedatory activity. The compounds of Formula 1 are useful as medicaments in mammals, including humans, for treatment of diseases and or alleviations of conditions which are responsive to treatment by agonists of alpha2 adrenergic receptors. Compounds of Formula 1 which have no significant cardiovascular and / or sedatory activity are useful for treating pain and other conditions with minimal side effects.
Owner:ALLERGAN INC
Use of selective antagonists of the alpha1B-adrenergic receptor for improvement of sexual dysfunction
InactiveUS6953800B2Increasing intracavernous pressureIncrease pressureCompound screeningBiocideSide effectHypotension shock
Described is the use in the treatment of either male or female sexual dysfunction of selective antagonists of the α1B-adrenergic receptor and the pharmaceutical compositions containing them as compounds capable of helping the sexual act avoiding at the same time excessive side effects due to acute hypotension.
Owner:RECORDATI CHEM & PHARMA
Compounds, Formulations and Methods for Reducing Skin Wrinkles, Creasing and Sagging
ActiveUS20110286945A1Cut skinEffective amountBiocideCosmetic preparationsΑ2 adrenergic receptorDermatology
Methods, compounds, and topical formulations for reduction of skin sagging, creasing and / or wrinkling are disclosed. The methods comprise topically applying a composition comprising an α2 adrenergic receptor agonist. Amelioration of skin sagging, creasing and / or wrinkling begins within minutes after topical application of a disclosed composition. A single application can significantly reduce skin sagging, creasing and / or wrinkling for at least about 8 hours.
Owner:GALDERMA LAB LP
4-(Phenylmethyl and substituted phenylmethyl)-imidazole-2-thiones acting as specific alpha2 adrenergic agonists
Compounds of Formula 1where the variables have the meaning defined in the specification are agonists of alpha2 adrenergic receptors. Several compounds of the disclosure are specific or selective to alpha2B and / or alpha2C adrenergic receptors in preference over alpha2A adrenergic receptors. Additionally some of the claimed compounds have no or only minimal cardivascular and / or sedatory activity. The compounds of Formula 1 are useful as medicaments in mammals, including humans, for treatment of diseases and or alleviations of conditions which are responsive to treatment by agonists of alpha2 adrenergic receptors. Compounds of Formula 1 which have no significant cardiovascular and / or sedatory activity are useful for treating pain and other conditions with minimal side effects.
Owner:ALLERGAN INC
Quaternary ammonium salt compounds
InactiveUS9072734B2High activityLower Level RequirementsOrganic chemistryRespiratory disorderPharmaceutical medicineBiology
Owner:TEIJIN PHARMA CO LTD
Method for treating psoriasis
InactiveUS8394800B2Treating and preventing psoriasisLittle side effectsBiocideInorganic non-active ingredientsΑ2 adrenergic receptorPharmacology
Methods and kits for treating or preventing psoriasis or a symptom associated with psoriasis in a subject are described. The methods involve topical applications to the subject a therapeutically effective amount of an α2 adrenergic receptor agonist, such as brimonidine.
Owner:GALDERMA LAB LP
Derivatives of 4-(2-amino-1-hydroxyethyl)phenol as agonists of the beta2 adrenergic receptors
The present disclosure relates to 4-(2-amino-1-hydroxyethyl)phenol derivatives of formula (I) as well as pharmaceutical compositions comprising them, and their use in therapy as agonists of the β2 adrenergic receptor.
Owner:ALMIRALL
5-(2--1-hydroxyethyl)-8-hydroxyquinolin-2(1H)-one and its use in the treatment of pulmonary diseases
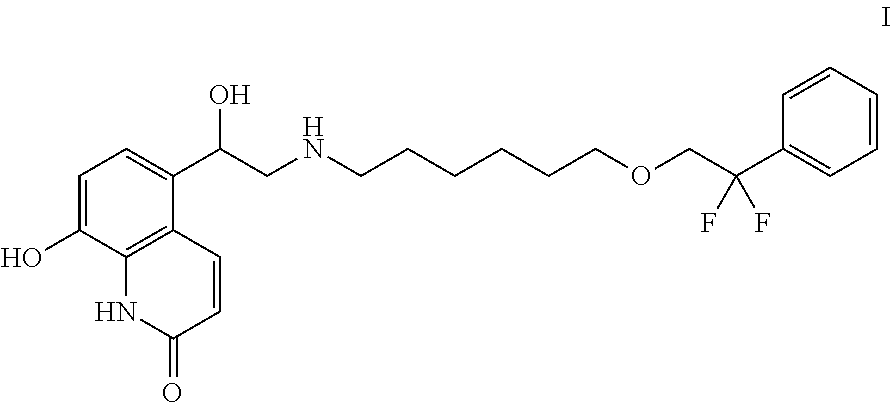
InactiveUS20120040941A1Good treatment effectSacrificing potencyBiocideNervous disorderMedicine8-Hydroxyquinoline
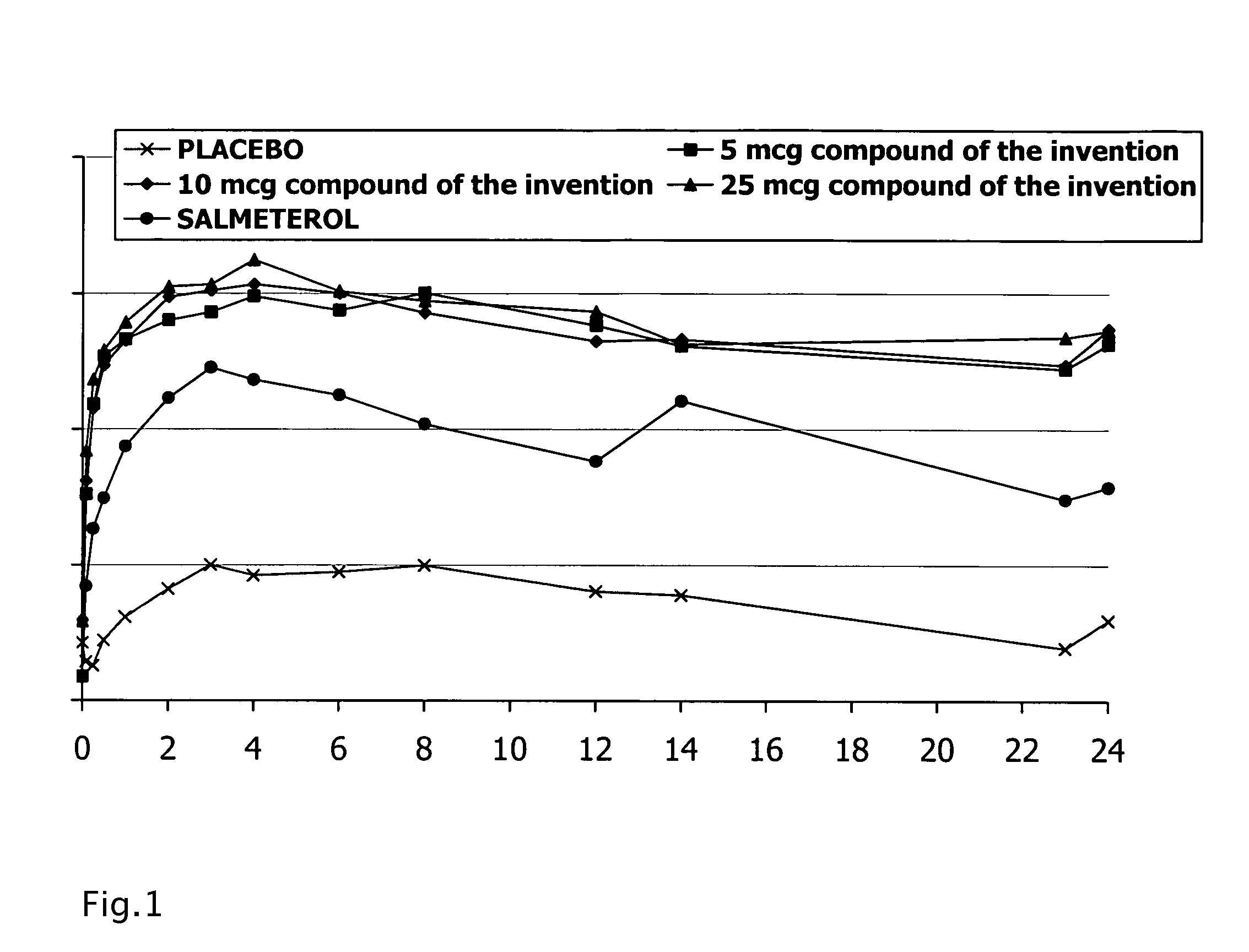
The present invention provides a compound, which is a hydroxyquinolinone derivative of formula (I),in the form of a racemate, a stereoisomer or a mixture of stereoisomers, or a pharmaceutically acceptable salt or solvate thereof, for use in a method of treating, for example, a pulmonary disease or condition associated with β2 adrenergic receptor activity in a mammal, in which the compound is administered by the inhalatory route at a metered nominal dose of less than 5 μg.
Owner:ALMIRALL
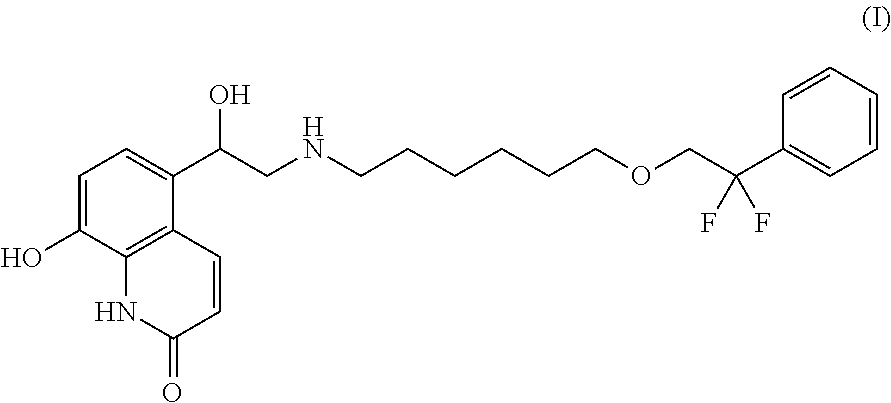
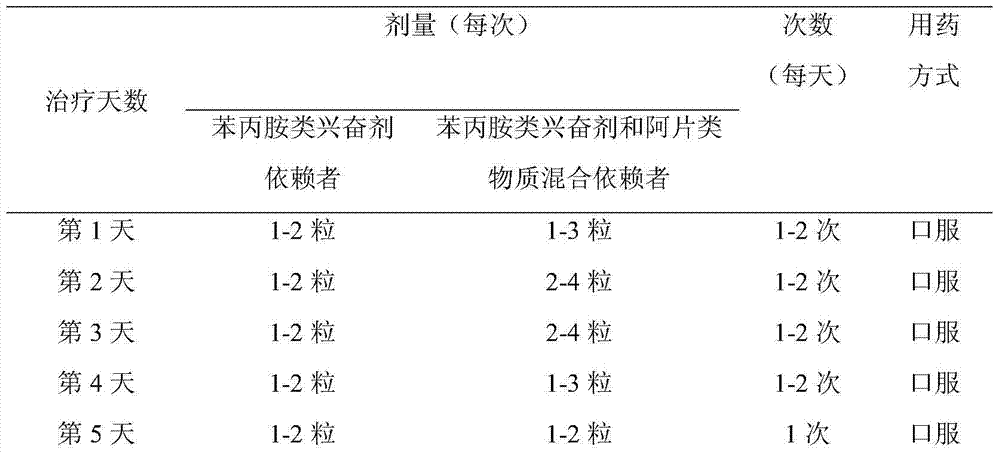
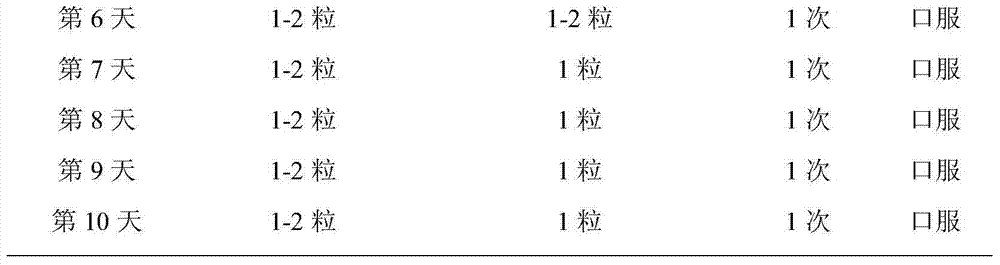
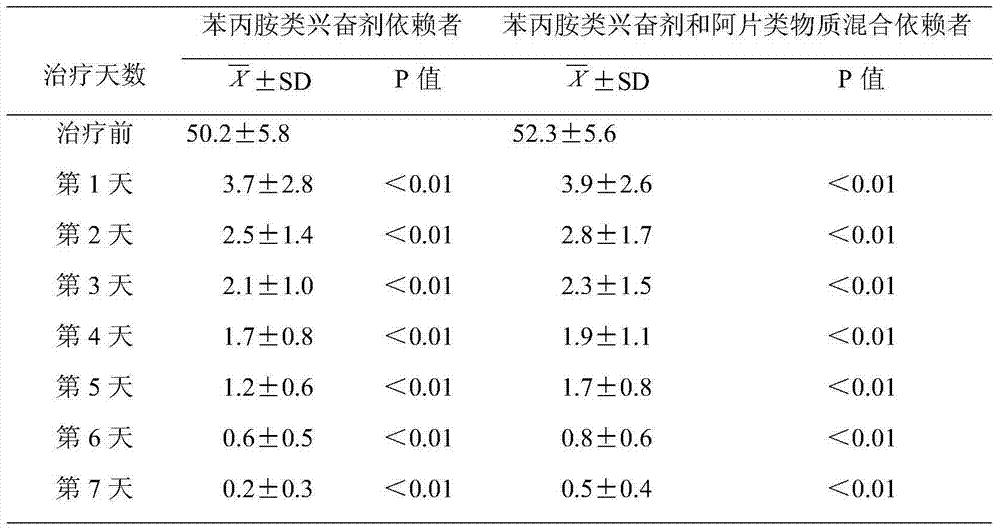
Medicament for treating amphetamine type stimulant dependency and mixed dependency of amphetamine type stimulants and opiates substances
InactiveCN103495172AGood effectControl withdrawal symptomsOrganic active ingredientsNervous disorderBenzodiazepineDrug withdrawal symptoms
The invention discloses a medicament for treating amphetamine type stimulant dependency and mixed dependency of amphetamine type stimulants and opiates substances. The medicament is prepared from the following components by weight percent: 2-95% of antipsychotics, 0.001-5% of alpha2 adrenergic agonists, 0-5% of anticholinergic agent, 0-80% of nonopioid analgesic, and 0-10% of benzodiazepine. The pharmaceutical composition disclosed by the invention achieves an ideal effect when the symptoms of a sufferer abusing stimulants such as benzedrine, or abusing the opiates substances in a merging manner are treated; the withdrawal symptom can be rapidly and obviously controlled; the detoxification recovery rate can be up to over 90%.
Owner:卢正堂 +1
Derivatives of 4-(2-amino-1-hydroxyethyl)phenol as agonists of the β2 adrenergic receptors
The present disclosure relates to 4-(2-amino-1-hydroxyethyl)phenol derivatives of formula (I) as well as pharmaceutical compositions comprising them, and their use in therapy as agonists of the β2 adrenergic receptor.
Owner:ALMIRALL
Beta2-adrenergic receptor agonists
InactiveUS7217738B2Rapid and efficient evaluationUrea derivatives preparationBiocideBovine respiratory diseaseAdrenergic
Disclosed are multibinding compounds which are β2 adrenergic receptor agonists and are useful in the treatment and prevention of respiratory diseases such as asthma, bronchitis. They are also useful in the treatment of nervous system injury and premature labor.
Owner:THERAVANCE BIOPHARMA R&D IP LLC
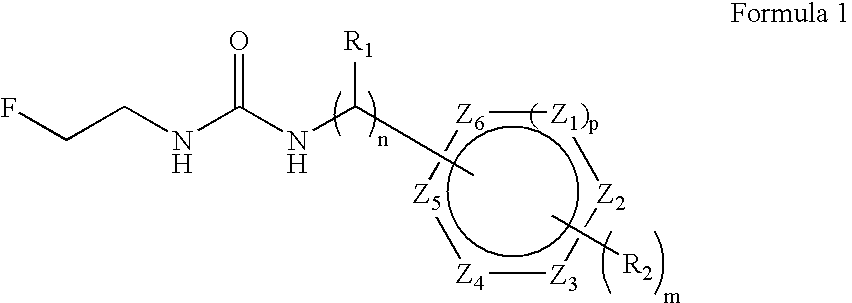
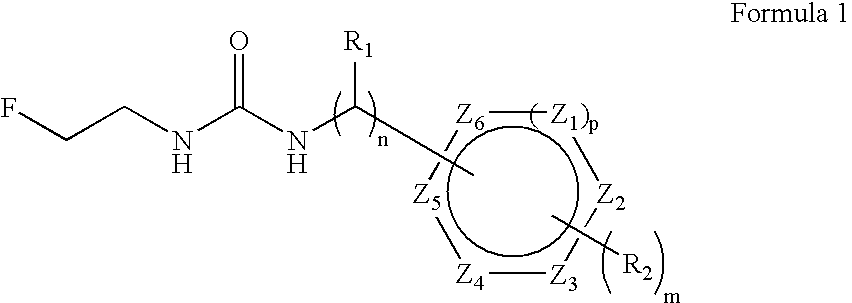
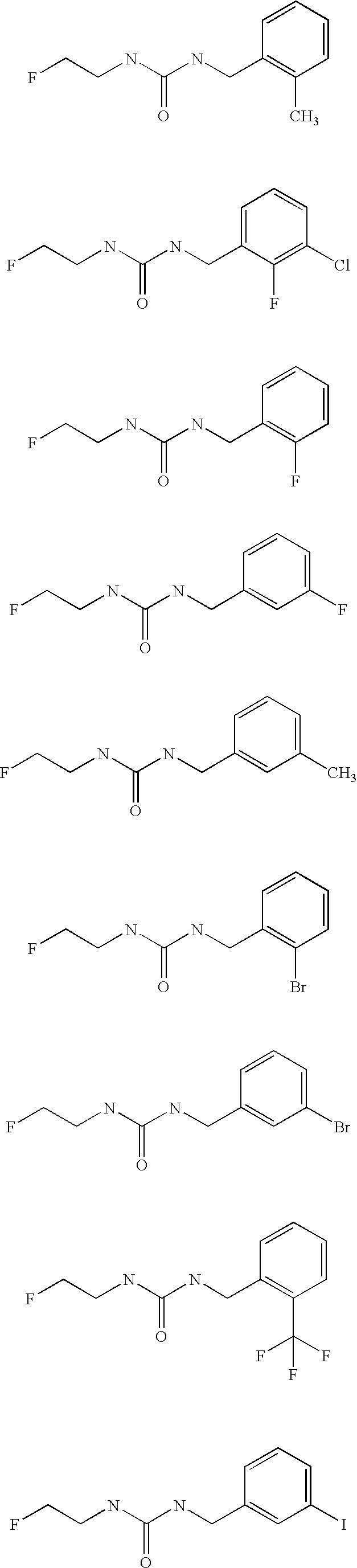
Aryl fluoroethyl ureas acting as alpha 2 adrenergic agents
The invention provides well-defined aryl fluoroethyl ureas that are useful as selective alpha2 adrenergic agonists. As such, the compounds described herein are useful in treating a wide variety of disorders associated with modulation of alpha2 adrenergic receptors.
Owner:ALLERGAN INC
Method for preventing or treating skin tumor
Methods, compositions and products for preventing skin tumor formation or inhibiting development of an existing skin tumor in a subject are described. The methods involve administering to the subject a composition containing an α2 adrenergic receptor agonist, such brimonidine.
Owner:GALDERMA RES & DEV SNC
4-(Condensed cyclicmethyl)-imidazole-2-thiones acting as α2 adrenergic agonists
Compounds of Formula 1where the variables have the meaning defined in the specification are agonists of alpha2 adrenergic receptors. Several compounds of the disclosure are specific or selective to alpha2B and / or alpha2C adrenergic receptors in preference over alpha2A adrenergic receptors. Additionally some of the claimed compounds have no or only minimal cardivascular and / or sedatory activity. The compounds of Formula 1 are useful as medicaments in mammals, including humans, for treatment of diseases and or alleviations of conditions which are responsive to treatment by agonists of alpha2 adrenergic receptors. Compounds of Formula 1 which have no significant cardiovascular and / or sedatory activity are useful for treating pain and other conditions with minimal side effects.
Owner:ALLERGAN INC
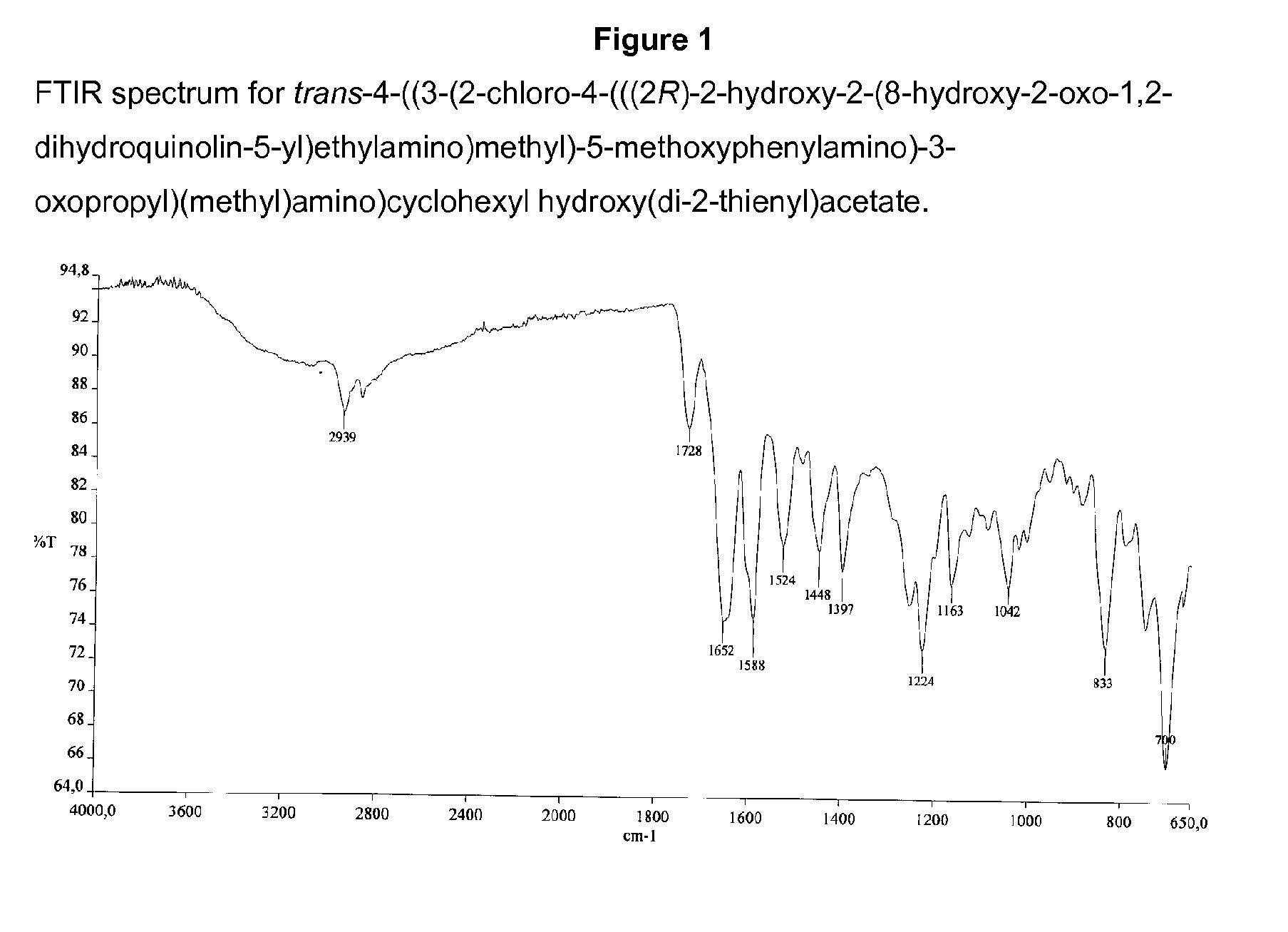
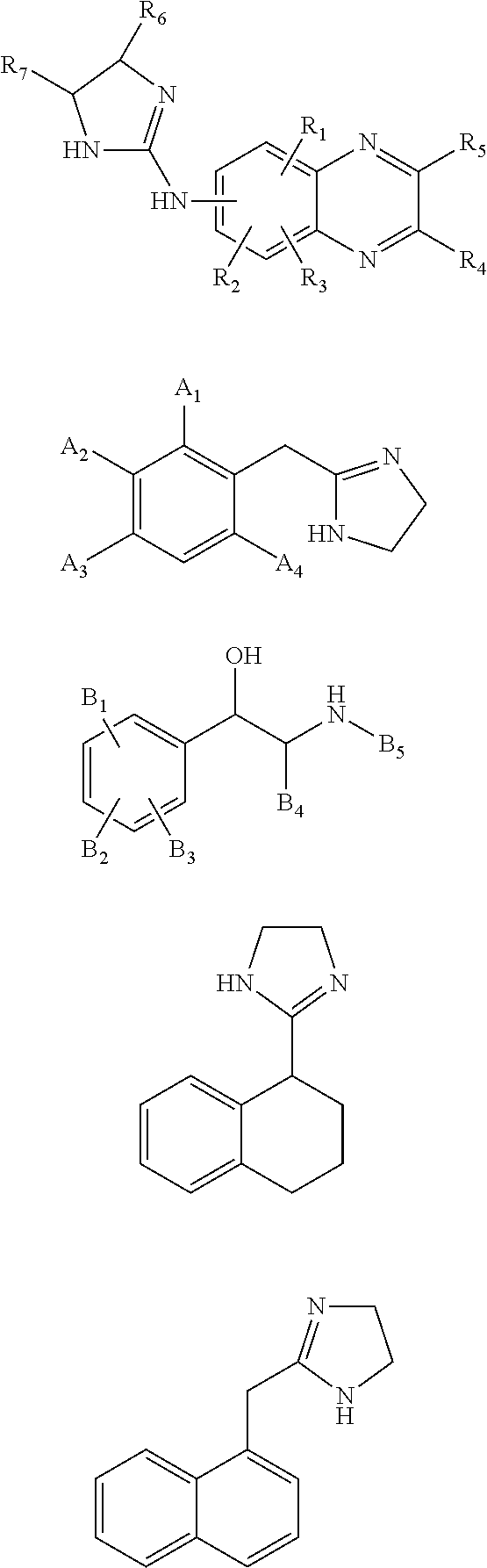
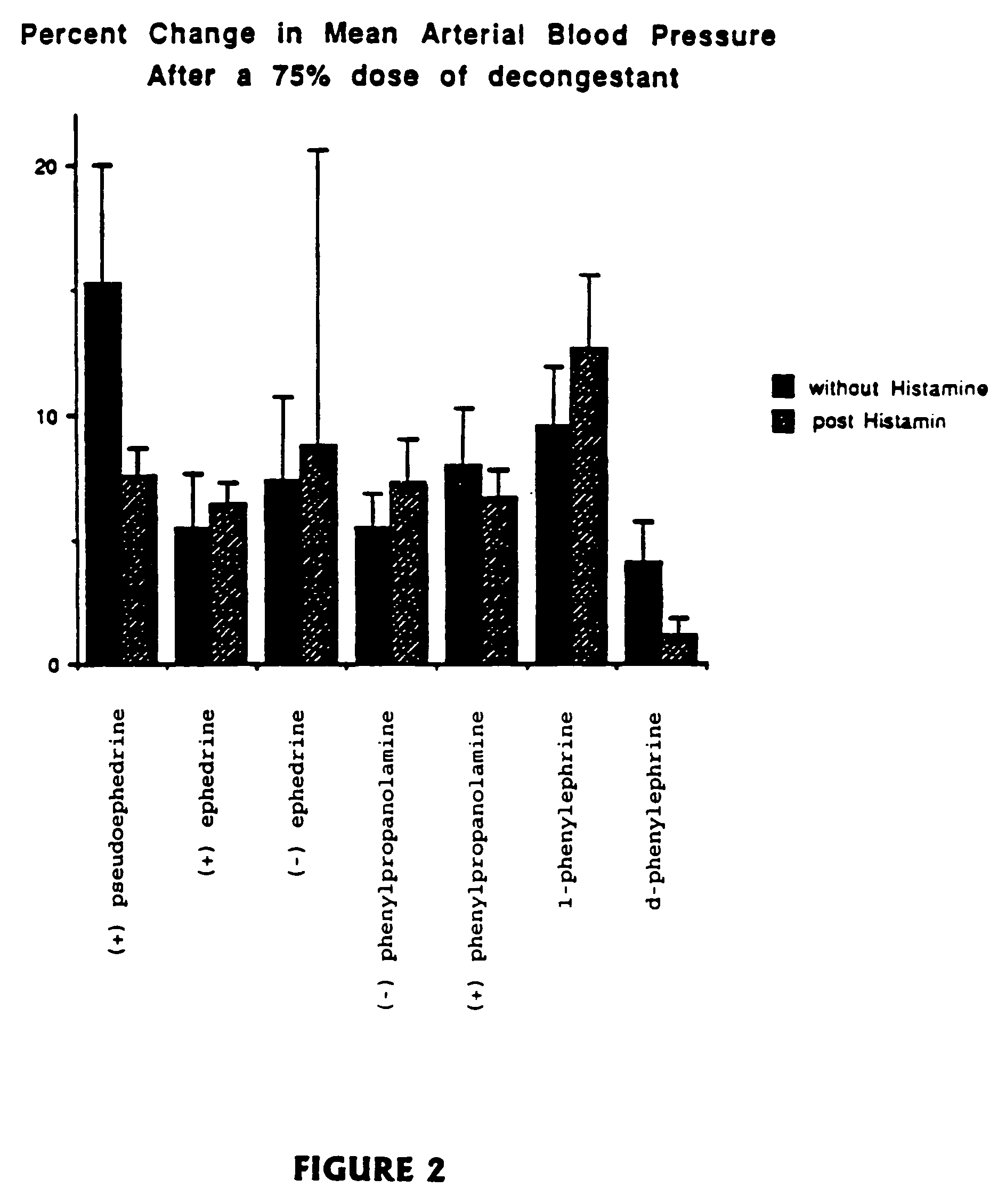
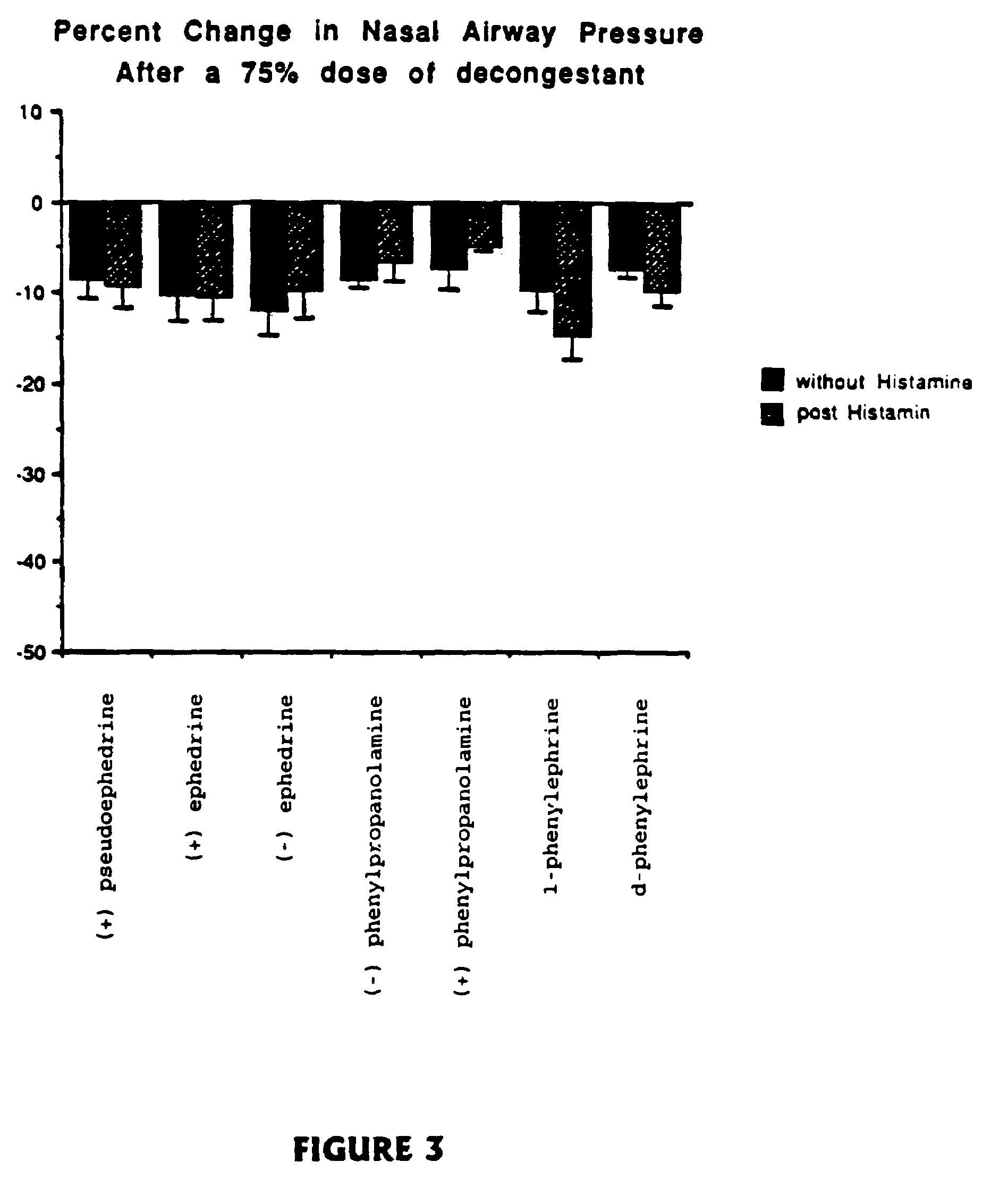
Stereoisomers with high affinity for adrenergic receptors
The present invention provides stereoscopically-pure diastereomers of Formula I:In a preferred embodiment, the stereoisomers of the present invention are of Formula II, depicted below:R2, R3 and R4 are independently H, OH, OCH3, CH2OH, NHCONH2, NH2, halogen or CF3, and R1 is pyridine, or an amine which may be substituted with hydrogen, lower alkyl, lower alkylenearyl, lower alkylenephenyl, lower alkylenehydroxyphenyl, lower alkyleneamine, lower alkyleneaminoaryl, lower alkylaminohydroxyphenyl, or a similar functional group. R5 is hydrogen, hydroxyl or methyl; R6 is hydrogen, lower alkyl, lower alkylenaryl, lower alkylenephenyl, lower alkylenehydroxyphenyl, lower alkyleneamine, lower alkyleneaminoaryl, lower alkylaminohydroxyphenyl, and the like. For both Formula I and Formual II, the first carbon on the side chain progressing from the ring is preferably in the R-configuration. The second carbon atom on the side chain of Formula II, which is attached to R5, may or may not be a chiral center. However, when the second carbon atom is a chiral center, it is preferably in the S-configuration. The present invention contemplates each stereoisomer of Formula I and II in substantially-pure form.The present invention also provides methods of relieving nasal, sinus and bronchial congestion and of treating attention deficit hyperactivity disorder and obesity. The present stereoisomers may also be used to induce pupil dilation. These methods include administering to a mammal a composition containing a therapeutically effective amount of a stereoscopically-pure stereoisomer of Formula I or II with a pharmaceutically acceptable excipient.
Owner:JOHNSON & JOHNSON CONSUMER COPANIES
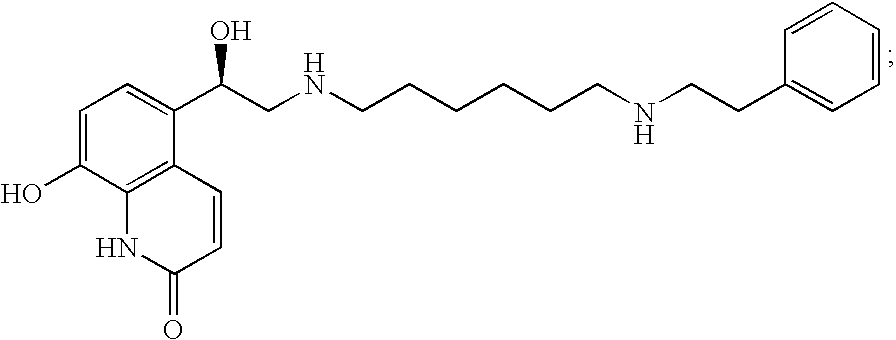
Diamine Beta2 Adrenergic Receptor Agonists
Owner:THERAVANCE BIOPHARMA R&D IP LLC
Functionally selective alpha2C adrenoreceptor agonists
In its many embodiments, the present invention provides a novel class of heterocyclic derivatives as a2C adrenergic receptor agonists, methods of preparing such compounds, pharmaceutical compositions containing one or more such compounds, methods of preparing pharmceutical formulations comprising one or more such compounds, and methods of treatment, prevention, inhibition, or amelioration of one or more conditions associated with the a2C adrenergic receptors using such compounds or pharmaceutical compositions.
Owner:MERCK SHARP & DOHME CORP
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com