Methods and compositions for treating celiac disease
a technology for celiac disease and composition, applied in the field of celiac disease treatment, can solve the problems of urgent need for non-dietary treatment alternatives and difficult maintenance of gluten-free diet, and achieve the effects of improving or ameliorating symptoms, preventing the onset of negative gastrointestinal reaction, and improving the condition of the subj
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
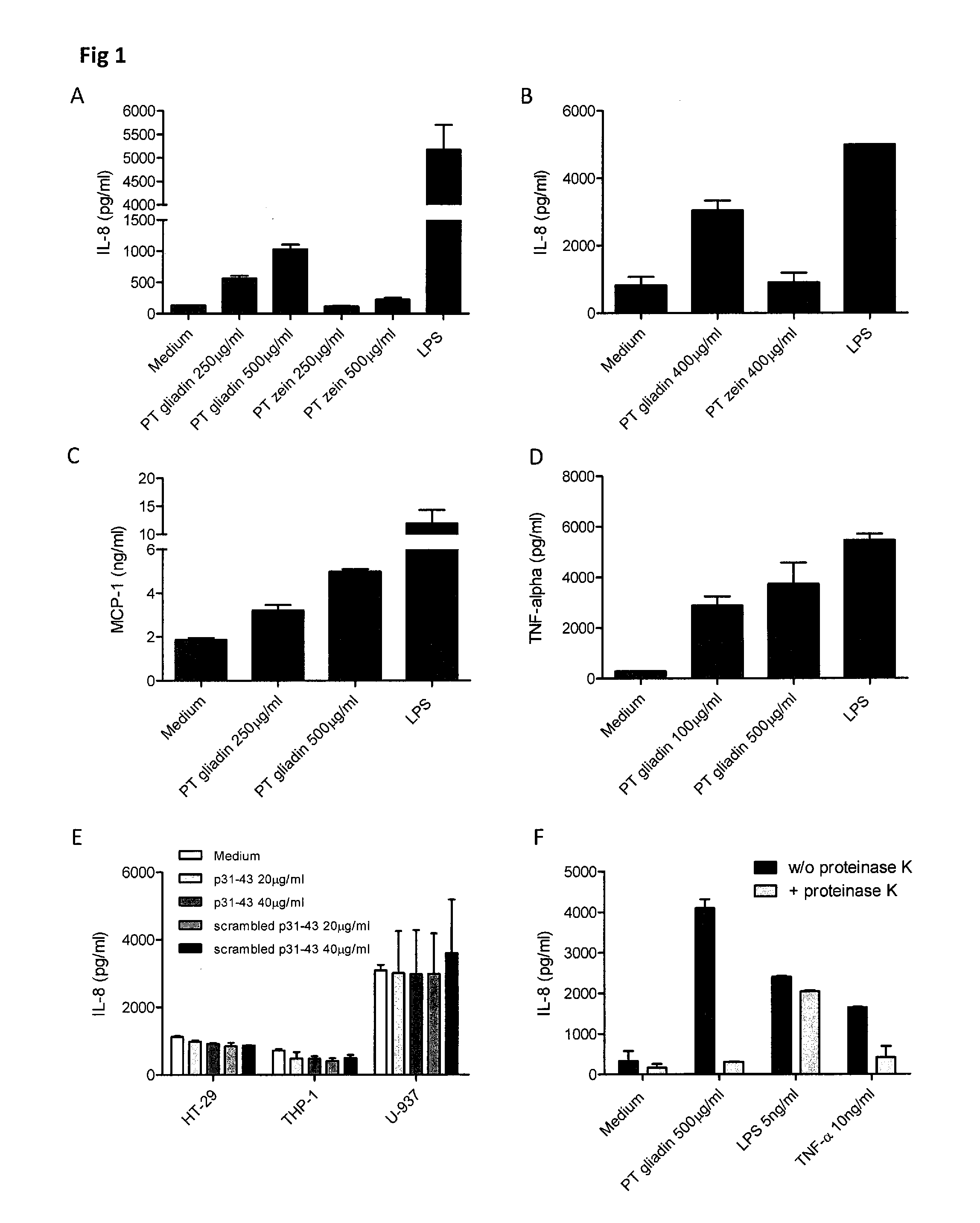
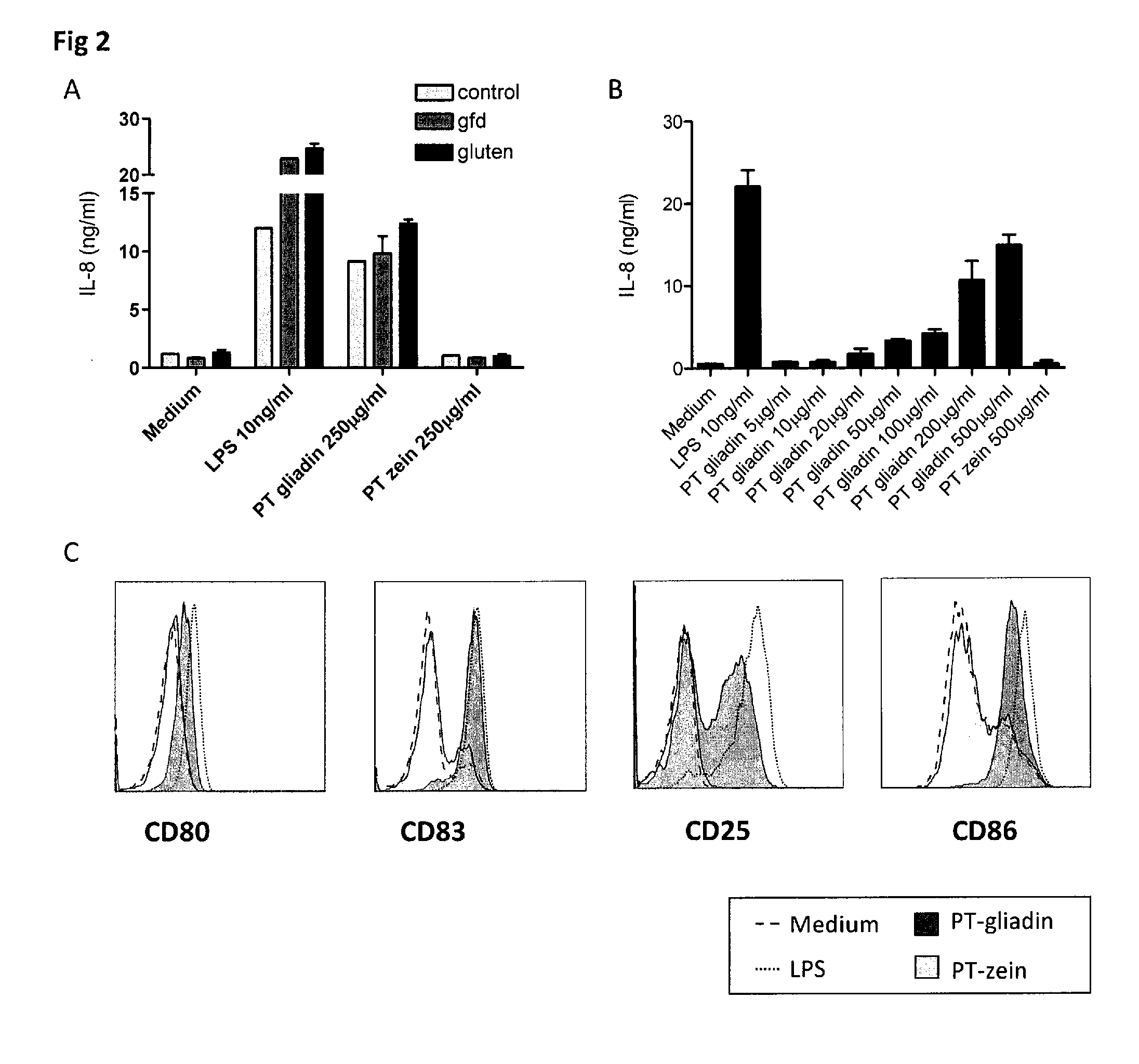
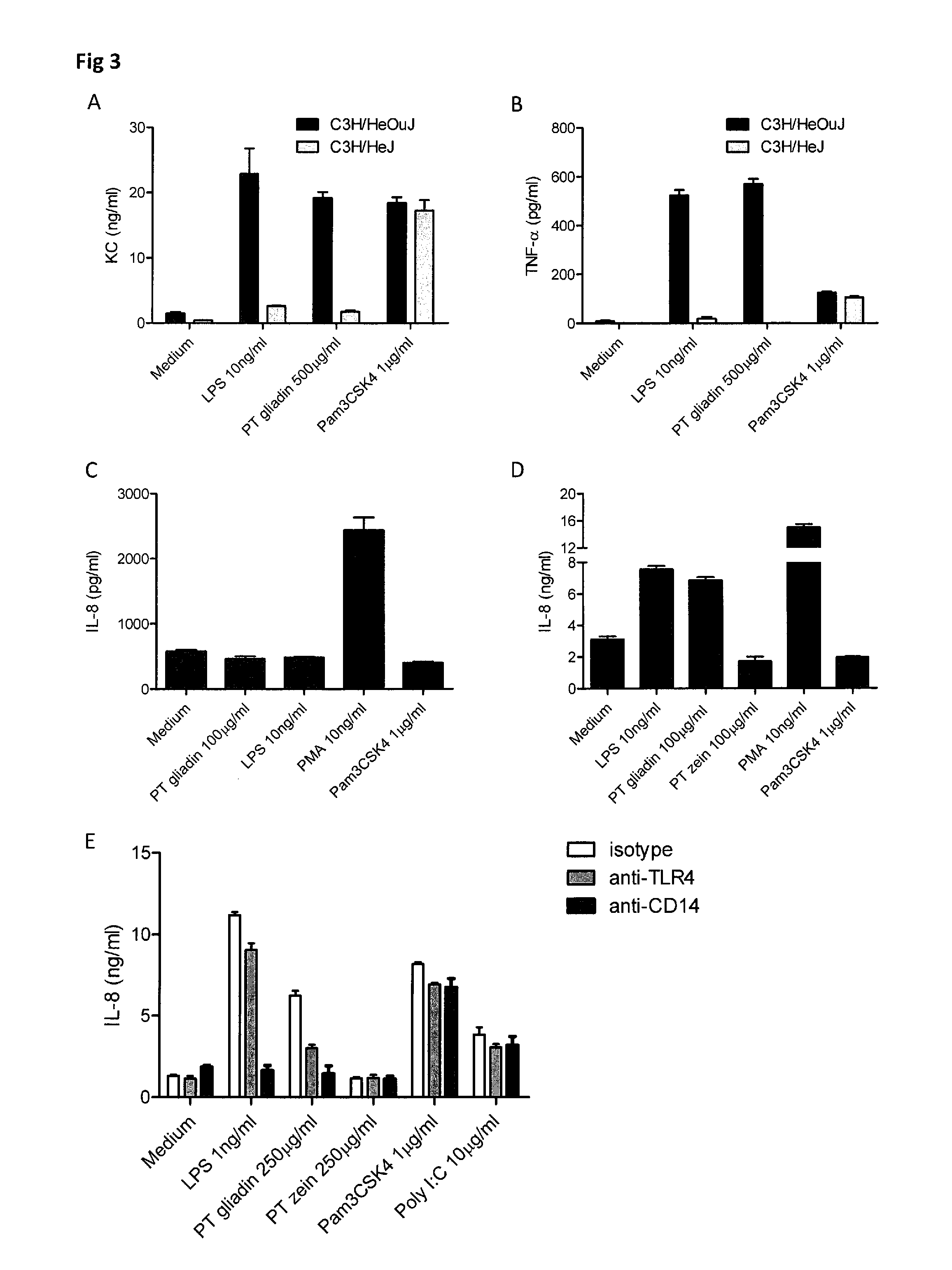
[0062]In general, the invention features the treatment of gastrointestinal disorders associated with an innate immune response triggered by alpha amylase inhibitor CM3, alpha amylase inhibitor 0.19 (0.19), CM1, CM2, CMa, CMd, CM16, CMb, CMX1 / CMX3, CMX2, alpha Amylase Inhibitor 0.53 (0.53) (collectively termed AAI), and other structurally and functionally related molecules. To this end, the invention provides pharmaceutical compositions including neutralizing antibodies to alpha amylase inhibitor CM3, alpha amylase inhibitor 0.19 (0.19), CM1, CM2, CMa, CMd, CM16, CMb, CMX1 / CMX3, CMX2, alpha Amylase Inhibitor 0.53 (0.53), and other structurally and functionally related molecules, food products containing reduced levels of AAI proteins, assays for identifying AAI protein content in food products, and assays for diagnosing subjects with a disorder related to AAI-triggered innate immune responses. The invention also features methods of treating gastrointestinal disorders associated with ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Composition | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com