Methods for measuring concentrations of biomolecules
a biomolecule and concentration technology, applied in the direction of instruments, assay labels, material analysis, etc., can solve the problems of increasing public health problems, loss of memory, cognitive function, independence and death, and disease that takes a heavy personal and financial toll on the patient, the family, and society
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
Stable Isotope Kinetic Labeling
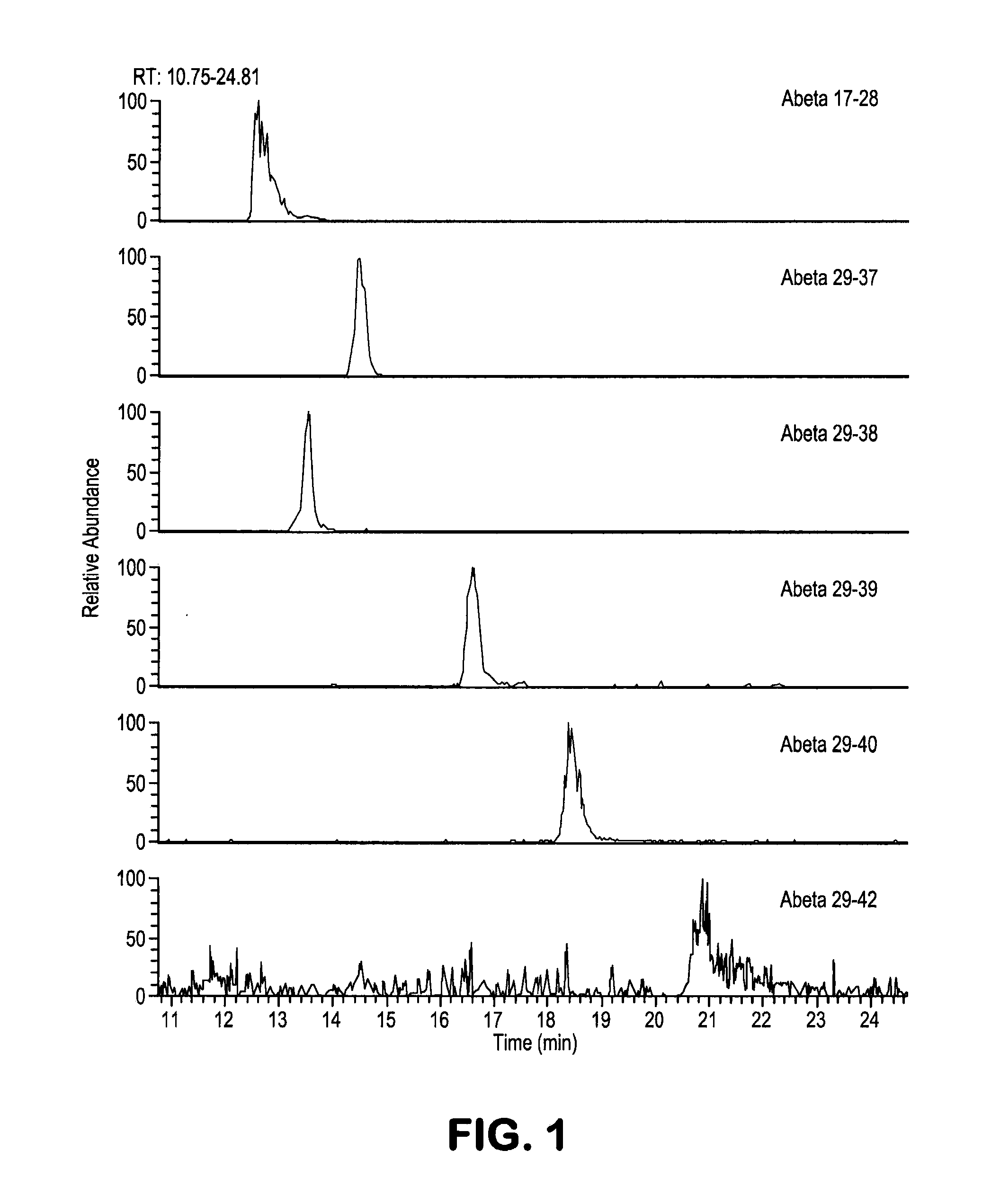
[0080]This example illustrates the improvements developed over the SILK methodology. A cell culture media was generated with a known ratio of labeled to unlabeled Aβ. This standard curve media was thereafter used to test the detection and quantitation of the 29-x peptides of Aβ. Using this media, a series of tests were performed to improve the solubility of the 29-x peptides as well as to establish improved chromatography conditions in order to increase detection of the peptides.
[0081]Increased Solubility of the 29-x Peptides—
[0082]The original SILK-Aβ™ methodology was modified by use of 0.025% of detergent, for example Tween-20, Triton, or CHAPS, instead of using 0.5M guanidine for the IP and washing steps. The peptides are kept in the 0.025% detergent solution during digestion as well and until injected into the mass spectrometer. Aβ is a very hydrophobic peptide and the addition of Tween-20 greatly improves the solubility and mass spectrometer signa...
example 2
Quantitation Standard
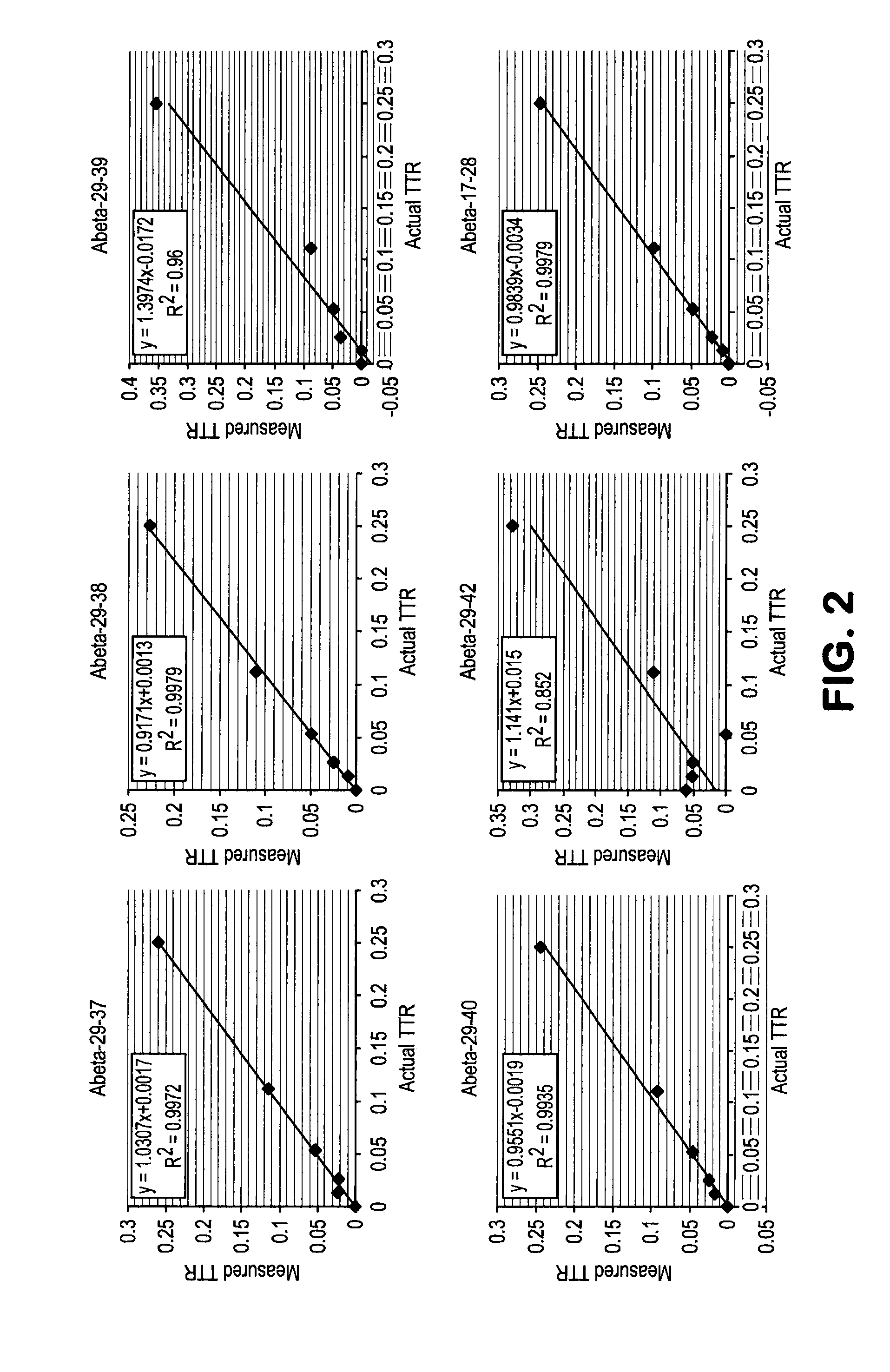
[0088]This example demonstrates that spiking of quantitation standard can be used for calculation of the ratio between endogenous peptides and spiked peptides. Thus, spiking in known amounts of labeled Aβ 37, Aβ 38, Aβ 39, Aβ 40 and Aβ 42 allows specific quantitation of those isoforms in addition to quantitation of the total Aβ concentration in the sample.
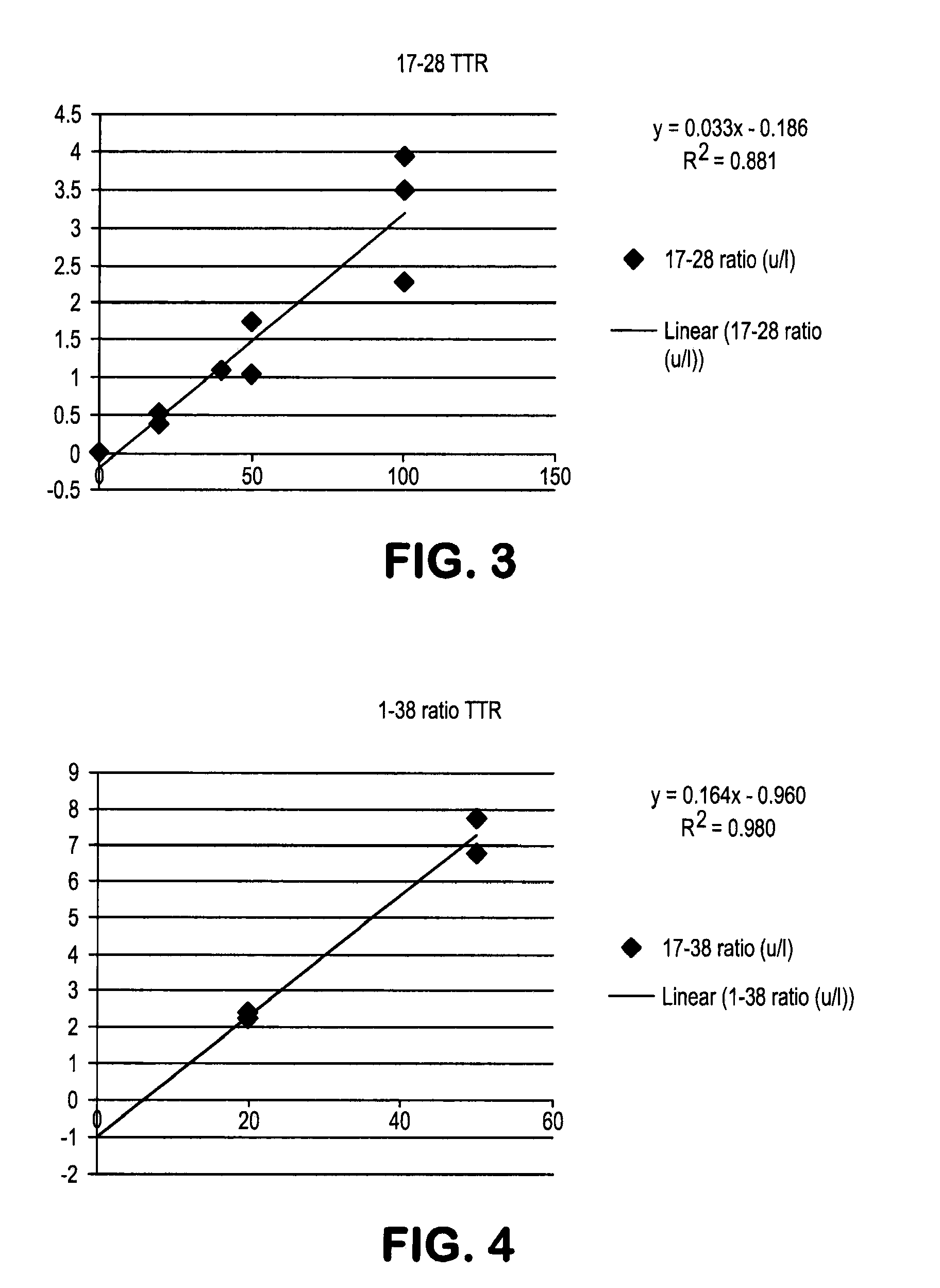
[0089]Due to the lead time of manufacturing labeled peptides we originally used unlabeled peptides as the spike in and used media from cells grown in 100% 13C6 leucine as the unknown. We spiked in unlabeled Aβ 38 and Aβ42 into 100% 13C6 leucine media and measured the ratios. As more unlabeled peptide was spiked into the media the tracer to tracee ratio (TTR, in this experiment defined as ratio of unlabeled to labeled) of both the 17-28 peptide and the 29-38 peptide increased linearly. (See FIGS. 3 and 4).
[0090]This illustrates the feasibility of measuring the concentration of these peptides based upon spiking a...
example 3
Quantitation Standard to Calculate Absolute Concentration
[0091]This example demonstrates use of the Quantitation Standard to calculate the production or clearance rates of biomolecules of interest, which is used to calculate absolute concentration of those biomolecules.
[0092]A sample containing Aβ or other peptide isoforms (including, but not limited to Aβ 1-37, Aβ 1-38, Aβ 1-39, Aβ 1-40, Aβ 1-42) in both unlabeled form, as well as labeled with 13C6-leucines (the labeled leucine is incorporated into the sample by the subject's natural biological processes) is spiked with Quantitation Standard. The ratio of labeled to unlabeled peptide over time is used to calculate kinetic production and clearance rates (metabolism) of the peptide in the subject. The ratios of labeled or unlabeled peptide to Quantitation Standard are used to calculate concentrations of the respective versions of the peptide.
[0093]The biomolecule (e.g., protein or peptide) is immunoprecipitated utilizing an antibody ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| time | aaaaa | aaaaa |
| time | aaaaa | aaaaa |
| time | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com