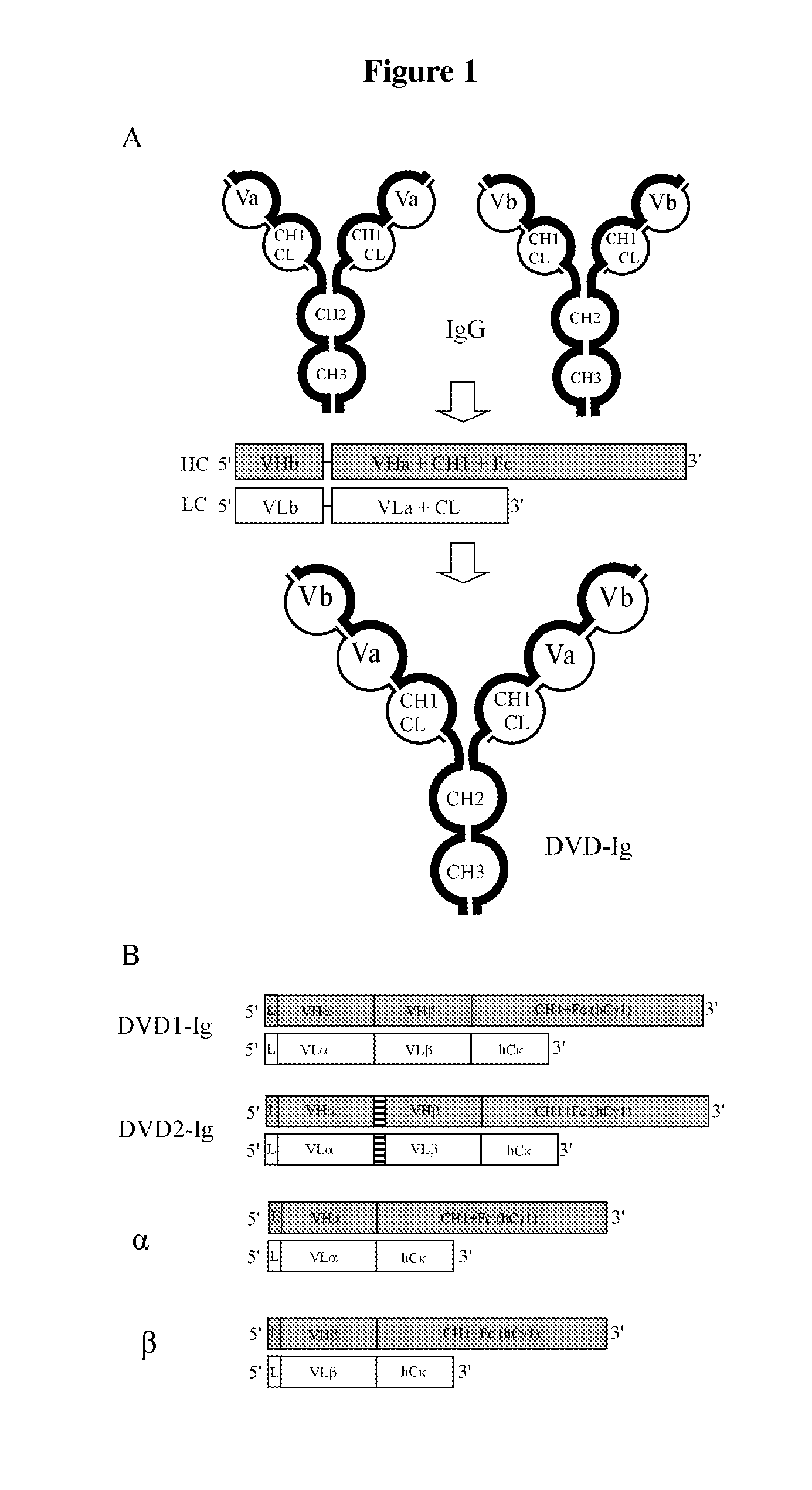
Bispecific immunobinders directed against TNF and il-17
a technology of immunobinders and il-17, which is applied in the field of bispecific immunobinders directed against tnf and il17, can solve the problems of reduced production yield, complex purification procedures, and inability to yield homogeneous preparations, and achieve the effect of reducing on
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
Preparation of Improved TNF-α Parental Binding Proteins
example 1.1
Identification of Fully Human Binding Proteins to TNF by In Vitro Display Systems
1.1.1: Antibody Selections
[0297]Fully human anti-human TNF monoclonal antibodies were isolated by in vitro display technologies from human antibody libraries by their ability to bind recombinant human TNF proteins. The amino acid sequences of the variable heavy (VH) and variable light (VL) chains were determined from DNA sequencing and listed in Table 1B in section A)I)A) above.
1.1.2: Affinity Maturation of the Fully Human Human TNF Binding Protein AE11-5
[0298]The AE11-5 human binding protein to human TNF was affinity matured by in vitro display technology. One light chain library was constructed to contain limited mutagenesis at the following residues: 28, 31, 32, 51, 55, 91, 92, 93, 95a and 96 (Kabat numbering). This library also contained framework germline back-mutations D1E, M4L, H11Q, R49K, H76N and Q103K as well as toggled residues at position 50 (R / K) and 94 (S / L) to allow for framework germlini...
example 1.2
Affinity Maturation of a Humanized Human TNF Binding Protein hMAK-195
[0301]The mouse anti-human TNF antibody MAK-195 was humanized and affinity-matured to generate a panel of humanized MAK195 variants that have cross-reactivity to cyno-TNF and improved affinity and binding kinetics against both human and cyno TNF.
[0302]To improve the affinity of hMAK195 to TNF, hypermutated CDR residues were identified from other human antibody sequences in the IgBLAST database that also shared high identity to germlines VH3-53 and IGKV1-39. The corresponding hMAK195 CDR residues were then subjected to limited mutagenesis by PCR with primers having low degeneracy at these positions to create three antibody libraries in the scFv format. The first library contained mutations at residues 31, 32, 33, 35, 50, 52, 53, 54, 56 and 58 in the VH CDR1 and 2 (Kabat numbering); the second library at residues 95 to 100, 100a, 101, and 102 in VH CDR3; and the third library at residues 28, 30, 31, 32, 50, 53, 92, 9...
PUM
| Property | Measurement | Unit |
|---|---|---|
| concentrations | aaaaa | aaaaa |
| molecular weight | aaaaa | aaaaa |
| dissociation constant | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com