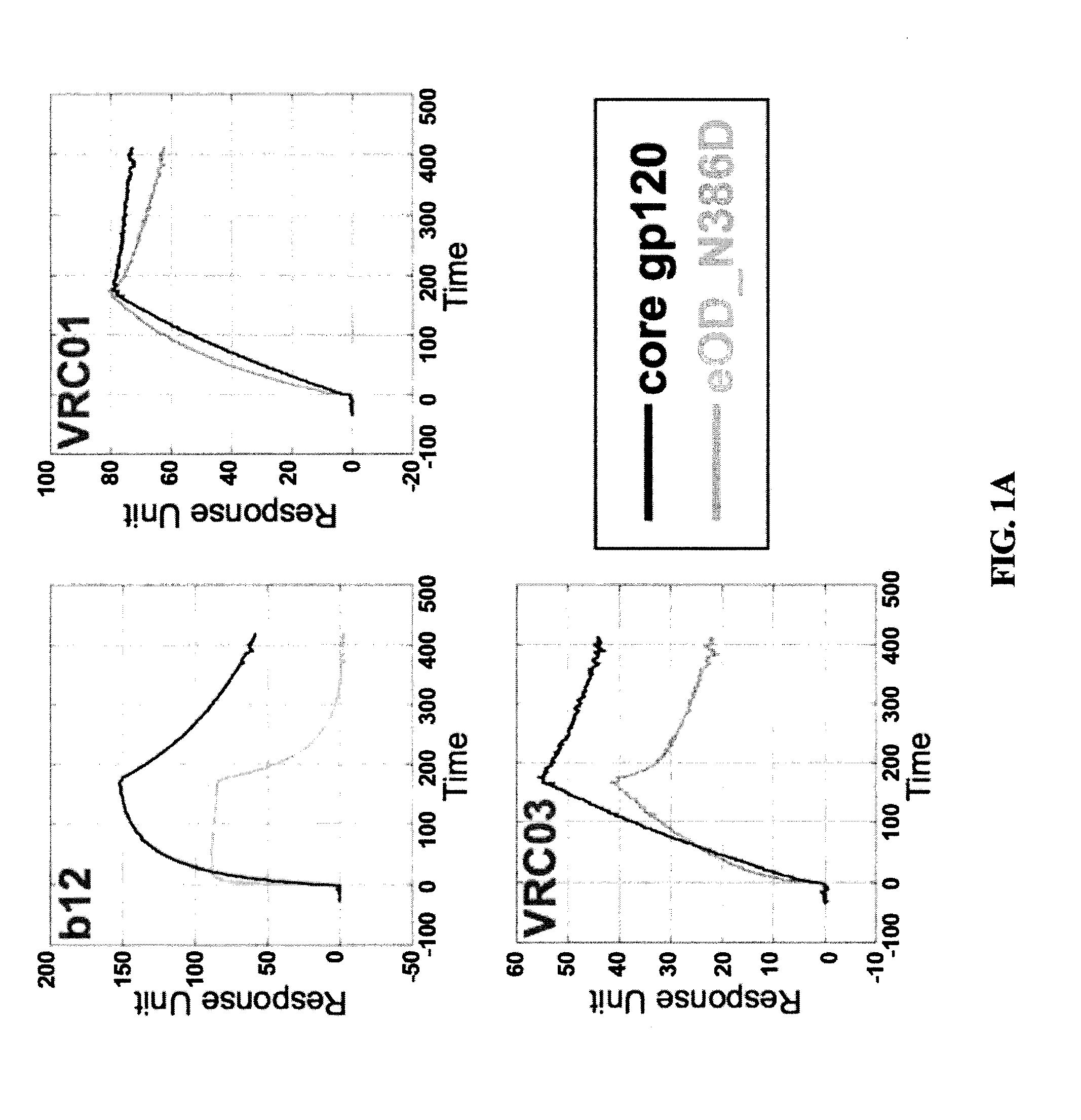
Engineered outer domain (EOD) of HIV gp120 and mutants thereof
a technology of outer domain and hiv gp120, which is applied in the field of engineered outer domain of hiv gp120 and mutants thereof, can solve the problems that cd4+ t cells seriously impair the body's ability to fight most invaders, and achieve the effect of improving the binding of germline vrc01
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
[0116]The invention pertains to the identification, design, synthesis and isolation of mutant eOD proteins disclosed herein as well as nucleic acids encoding the same. The present invention also relates to homologues, derivatives and variants of the sequences of the mutant eOD proteins and nucleic acids encoding the same, wherein it is preferred that the homologue, derivative or variant have at least 50%, at least 60%, at least 70%, at least 75%, at least 80%, at least 85%, at least 90%, at least 93%, at least 95%, at least 97%, at least 98% or at least 99% homology or identity with the sequence of the mutant eOD proteins and nucleic acids encoding the same. It is noted that within this specification, homology to sequences of the mutant proteins and nucleic acids encoding the same refers to the homology of the homologue, derivative or variant to the binding site of the mutant proteins and nucleic acids encoding the same.
[0117]The invention still further relates to nucleic acid seque...
PUM
| Property | Measurement | Unit |
|---|---|---|
| inhibitory concentration | aaaaa | aaaaa |
| OD | aaaaa | aaaaa |
| OD | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com