Detecting and quantifying cryptic HIV replication
a human immunodeficiency virus and cryptic technology, applied in the field of detection and quantification of cryptic hiv replication, can solve the problems of not being able to drive the evolution of antiviral resistance by itself, and not eliminating hiv completely from patients,
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
Detecting and Quantifying Cryptic HIV Replication from Measured 2-LTR Dynamics Following Raltegravir Intensification
1. Introduction
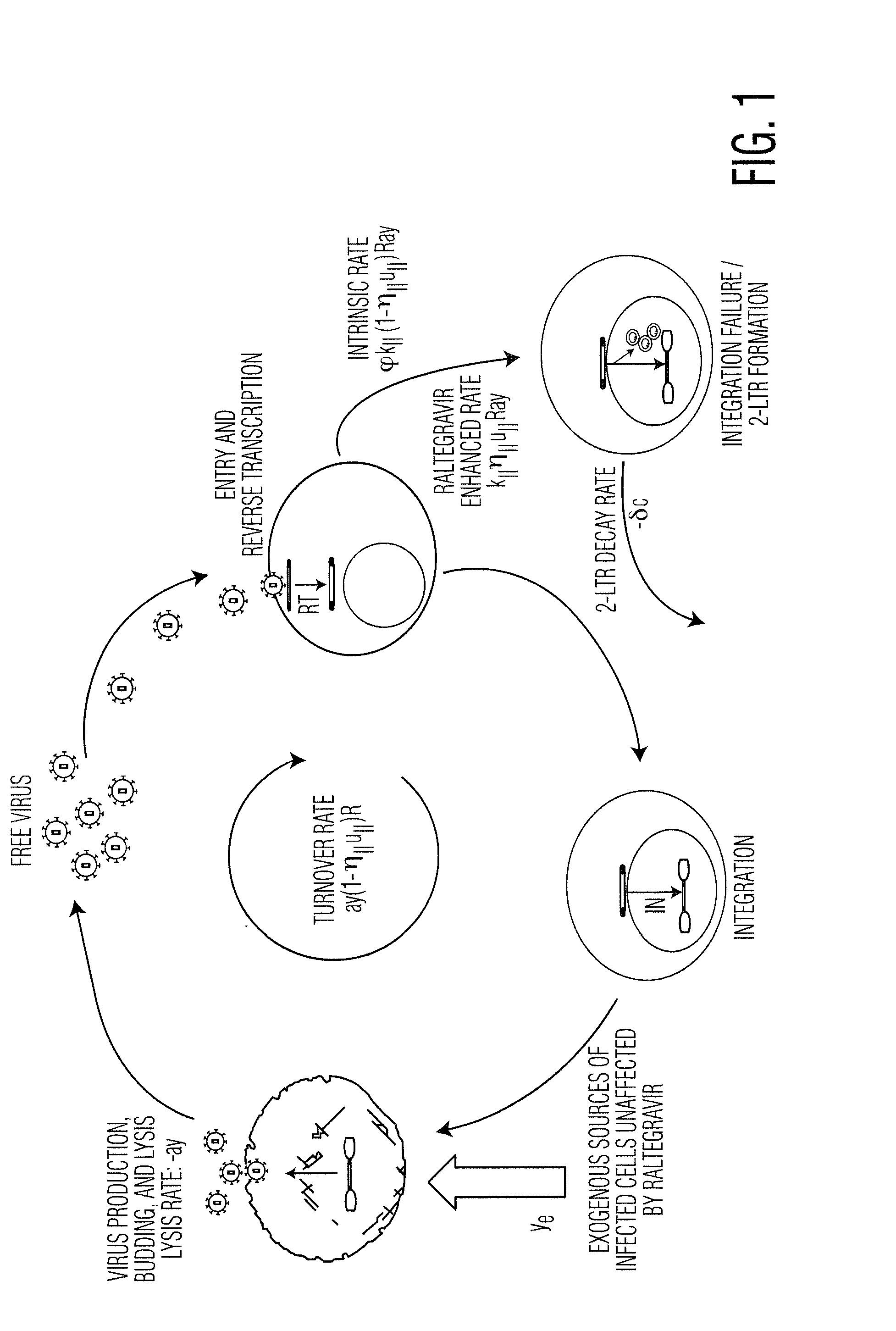
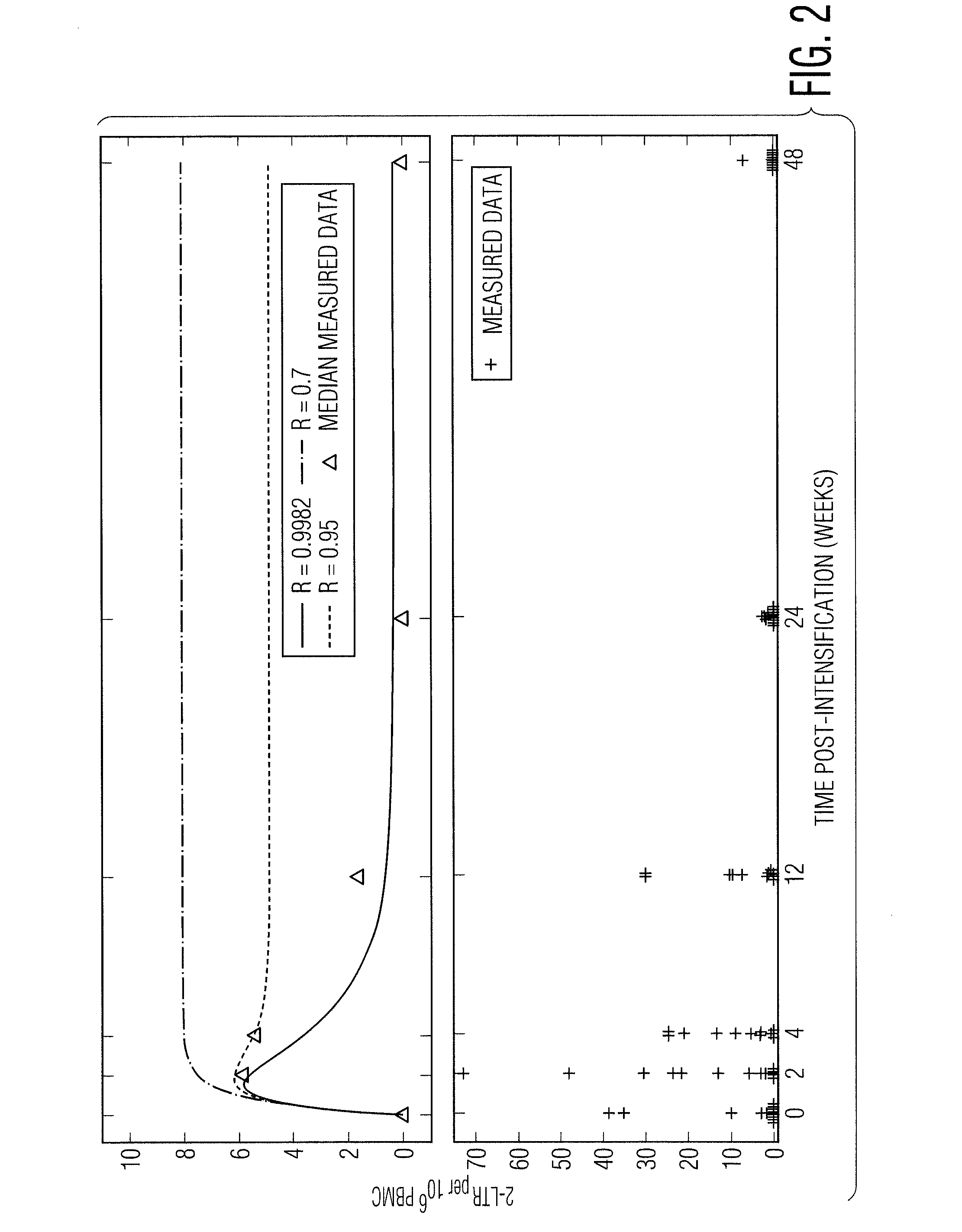
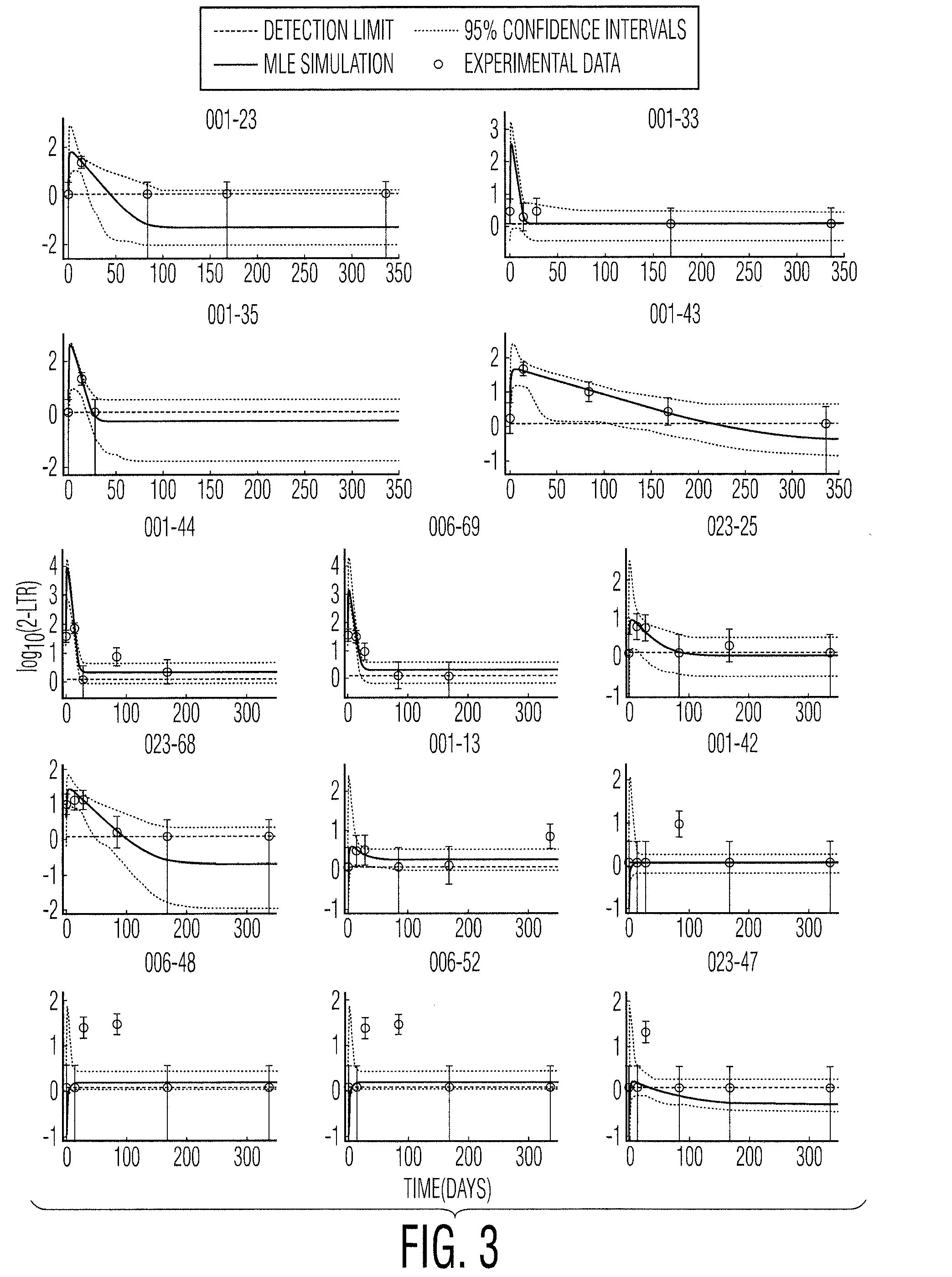
[0055]In a recently published INTEGRAL study, 45 patients on HAART who had maintained plasma viremia undetectable by standard assays for at least 1 year received standard HAART intensified by the addition of raltegravir for 48 weeks. During this time, Peripheral-Blood Mononuclear Cell (PBMC) samples were analyzed for the presence of cells containing 2-LTR circles. 2-LTR circles are formed when the linear viral DNA is prevented from integrating into the host cell genome, either through failed integration or through the action of integrase inhibitors such as raltegravir. It is expected, therefore, that the numbers of 2-LTR containing cells would increase if the raltegravir was interrupting otherwise successful infection events. 2-LTR containing cells were observed in 13 / 45 patients receiving raltegravir intensification, compared to 1 / 22 patients in the con...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Time | aaaaa | aaaaa |
| Time | aaaaa | aaaaa |
| Concentration | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com