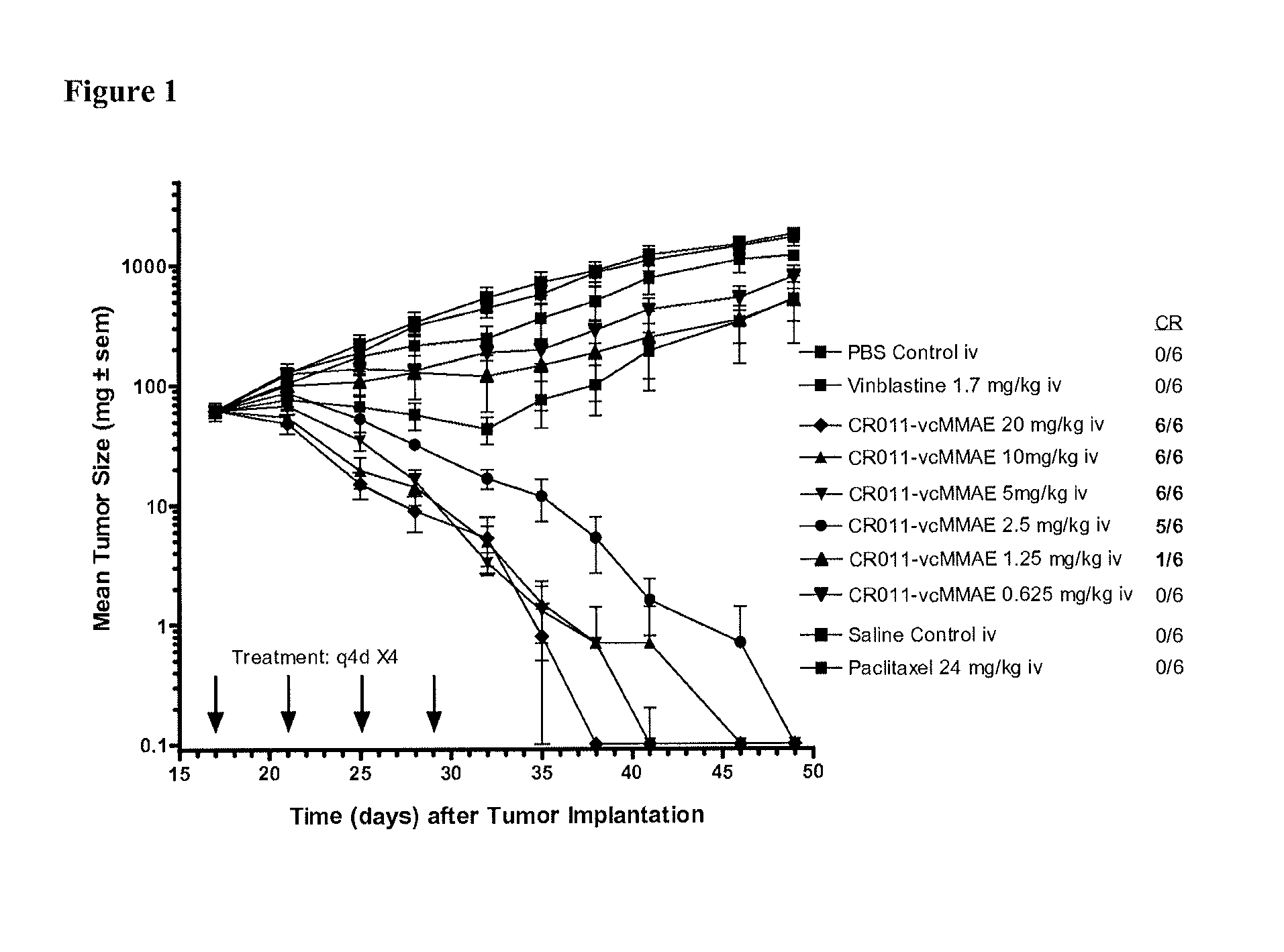
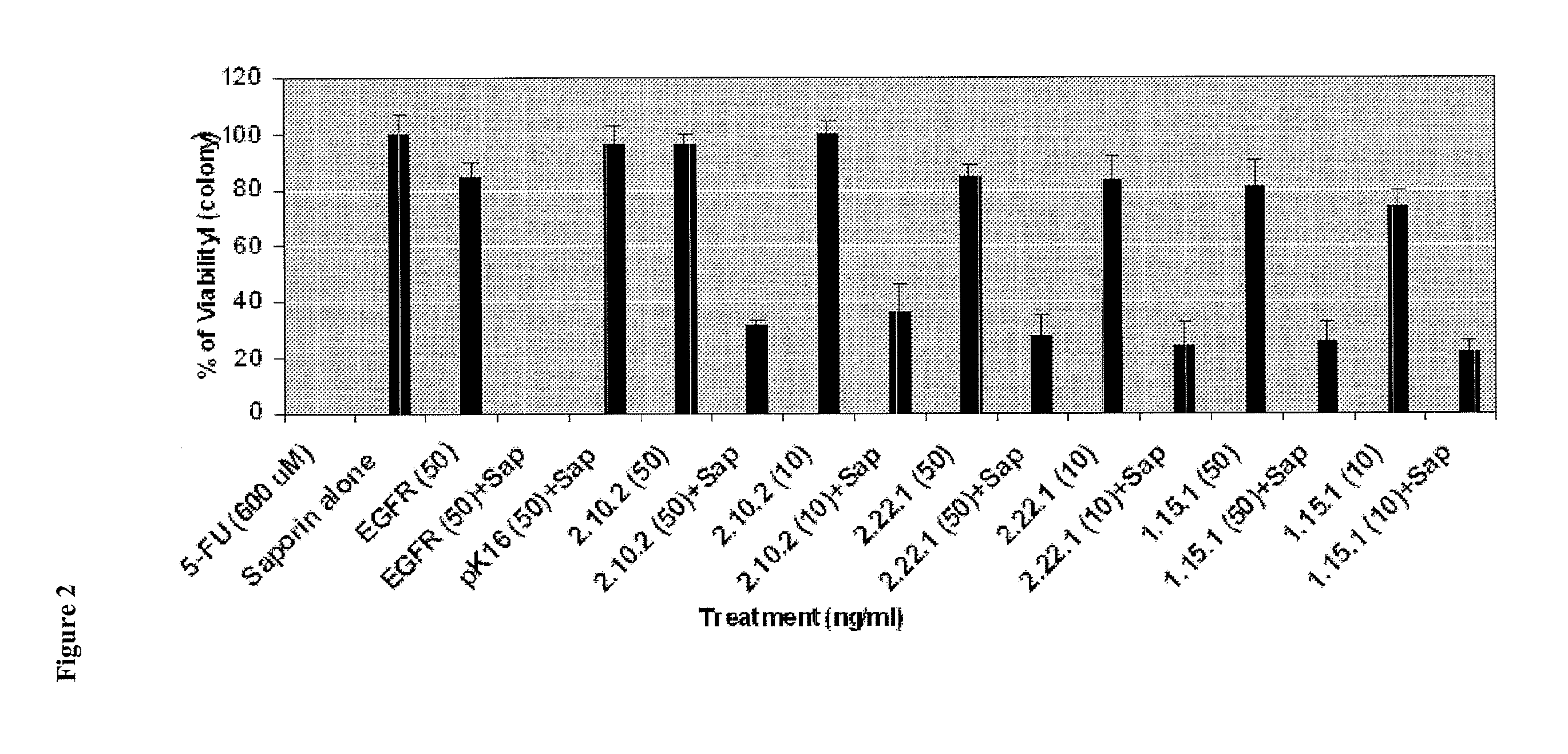
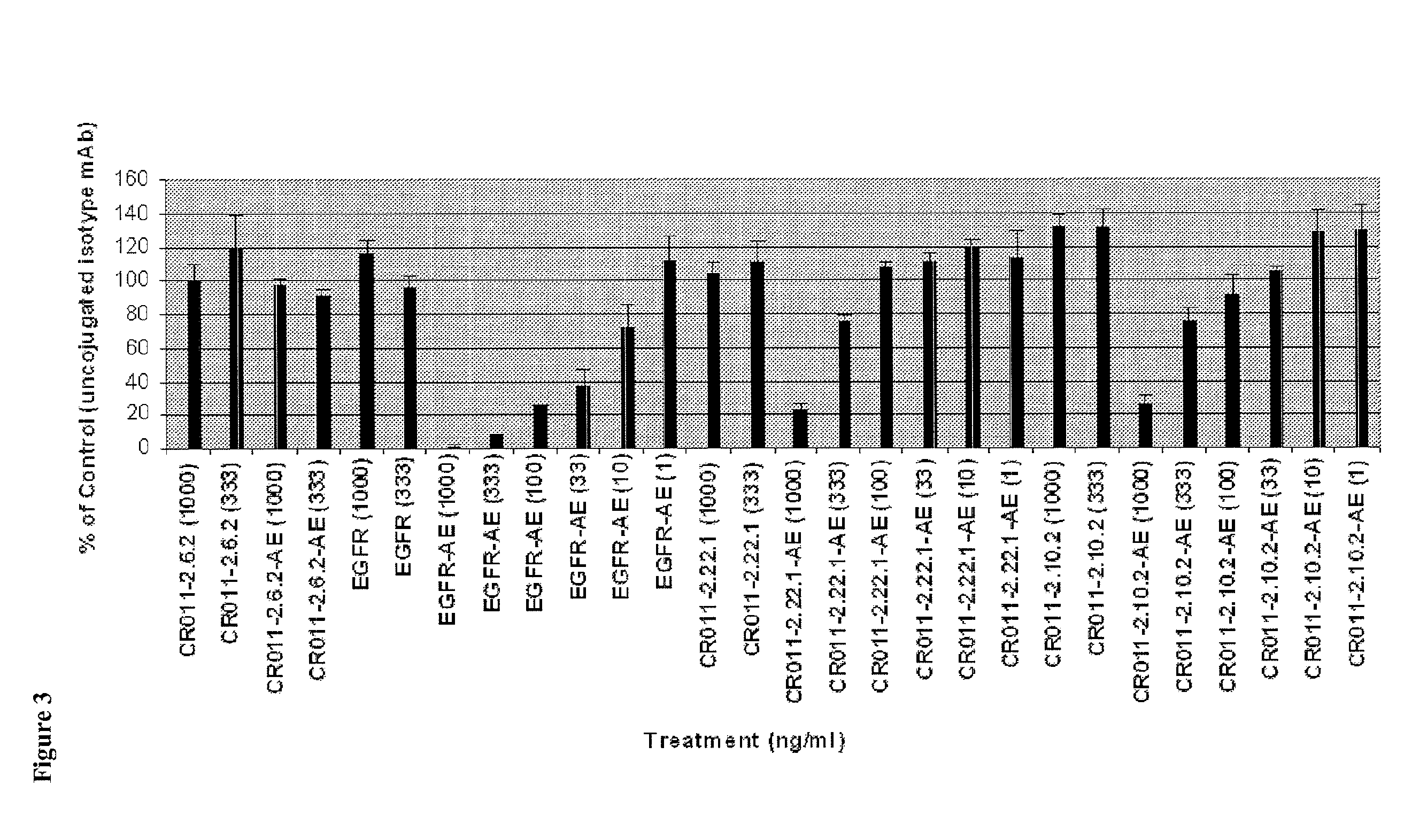
Antibodies Directed to GPNMB and Uses Thereof
a technology of gpnmb and antibodies, applied in the field of antibodies with specificity to gpnmb, can solve the problems of loss or addition of residues at the junction, approach, short half-life in vivo, and inefficient effector functions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
[0233]Recombinant human GPNMB (SEQ ID NO:289), specifically the extra-cellular domain (ECD) was prepared for use as the immunogen. Generally, cDNA encoding the ECD of GPNMB with a C-terminus V5-HIS tag was transfected into HEK 293 cells, expressed and purified using cation exchange chromatography with a POROS HS 50 (Applied Biosystems, Foster City, Calif.). Sample was eluted with 1M NaCl at a pH of 5.5, followed by metal affinity chromatography (Pharmacia metal chelate 5 mL). The sample was eluted against a linear gradient from 10-500 mM imidazole over 10 CV (column volumn). Dialysis occurred using 20 mM Tris / 50 mM NaCl at pH 7.4 (2 L×2). The sample was then filtered through a 0.22 μm filter.
example 2
Immunization
[0234]A preferred method for generating fully human antibodies uses XenoMouse® strains of mice which have been engineered to contain 245 kb and 190 kb-sized germline configuration fragments of the human heavy chain locus and kappa light chain locus (Green et al. 1994 Nature Genetics 7:13-21; Mendez et al. 1997 Nature Genetics 15:146-156; Green and Jakobovits, 1998 J. Exp. Med. 188:483-495; U.S. Pat. Nos. 6,162,963, 6,150,584, 6,114,598, 6,075,181, and 5,939,598). In an alternative approach, the minilocus approach, an exogenous Ig locus is mimicked through the inclusion of pieces (individual genes) from the Ig locus. Thus, one or more VH genes, one or more DH genes, one or more JH genes, a mu constant region, and a second constant region (preferably a gamma constant region) are formed into a construct for insertion into an animal (Taylor et al., 1992, Chen et al., 1993, Tuaillon et al., 1993, Choi et al., 1993, Lonberg et al., (1994), Taylor et al., (1994), and Tuaillon e...
example 3
Antibodies
[0243]Hybridoma cell lines were generated from immunized mice demonstrated to have anti-GPNMB titers using standard techniques (see Mendez et al, 1997, Nat. Genet. 15:146-156).
[0244]Immunized mice were sacrificed by cervical dislocation, and the lymph nodes were harvested and pooled from each cohort. The lymphoid cells were dissociated by grinding in DMEM to release the cells from the tissues, and the cells were suspended in DMEM. The cells were counted, and 0.9 mL DMEM per 100 million lymphocytes was added to the cell pellet to resuspend the cells gently but completely. Using 100 μl of CD90+ magnetic beads per 100 million cells, the cells were labeled by incubating the cells with the magnetic beads at 4° C. for 15 minutes. The magnetically-labeled cell suspension containing up to 108 positive cells (or up to 2×109 total cells) was loaded onto a LS+ column and the column washed with DMEM. The total effluent was collected as the CD90-negative fraction (most of these cells w...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com