Combination therapy of antibodies against human CSF-1R and antibodies agains human PD-L1
a technology of pd-l1 and csf-1r, which is applied in the field of csf1r and csf1r antibodies, can solve the problems of significant unmet medical needs, refractory, exhaustion or tolerance to foreign antigens, etc., and achieves the effect of efficient antiproliferative activity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
Inhibition of CSF-1-Induced CSF-1R Phosphorylation in NIH3T3-CSF-1R Recombinant Cells
[0453]4.5×103 NIH 3T3 cells, retrovirally infected with an expression vector for full-length CSF-1R, were cultured in DMEM (PAA Cat. No.E15-011), 2 mM L-glutamine (Sigma, Cat. No. G7513, 2 mM Sodium pyruvate, 1× nonessential amino acids, 10% FKS (PAA, Cat. No. A15-649) and 100 μg / ml PenStrep (Sigma, Cat. No. P4333 [10 mg / ml]) until they reached confluency. Thereafter cells were washed with serum-free DMEM media (PAA Cat. No. E15-011) supplemented with sodium selenite [5 ng / ml] (Sigma, Cat. No. S9133), transferrin [10 μg / ml] (Sigma, Cat. No. T8158), BSA [400 μg / ml] (Roche Diagnostics GmbH, Cat. No. 10735078), 4 mM L-glutamine (Sigma, Cat. No. G7513), 2 mM sodium pyruvate (Gibco, Cat. No. 11360), 1× nonessential amino acids (Gibco, Cat: 11140-035), 2-mercaptoethanol [0.05 mM] (Merck, Cat. No. M7522), 100 μg / ml and PenStrep (Sigma, Cat. No. P4333) and incubated in 30 μl of the same medium for 16 hours ...
example 2
Growth Inhibition of NIH3T3-CSF-1R Recombinant Cells in 3D Culture Under Treatment with Anti-CSF-1R Monoclonal Antibodies (CellTiterGlo-Assay)
[0455]NIH 3T3 cells, retrovirally infected with either an expression vector for full-length wildtype CSF-1R (SEQ ID NO: 62) or mutant CSF-1R L301S Y969F (SEQ ID NO: 63), were cultured in DMEM high glucose media (PAA, Pasching, Austria) supplemented with 2 mM L-glutamine, 2 mM sodium pyruvate and non-essential amino acids and 10% fetal bovine serum (Sigma, Taufkirchen, Germany) on poly-HEMA (poly(2-hydroxyethylmethacrylate)) (Polysciences, Warrington, Pa., USA)) coated dishes to prevent adherence to the plastic surface. Cells are seeded in medium replacing serum with 5 ng / ml sodium selenite, 10 mg / ml transferrin, 400 μg / ml BSA and 0.05 mM 2-mercaptoethanol. When treated with 100 ng / ml hu CSF-1 (active 149 aa fragment of human CSF-1 (aa 33-181 of SEQ ID NO: 86); Biomol, DE, Cat. No. 60530) wtCSF-1R (expressing cells form dense spheroids that gro...
example 3
Inhibition of Human Macrophage Differentiation Under Treatment with Anti-CSF-1R Monoclonal Antibodies (CellTiterGlo-Assay)
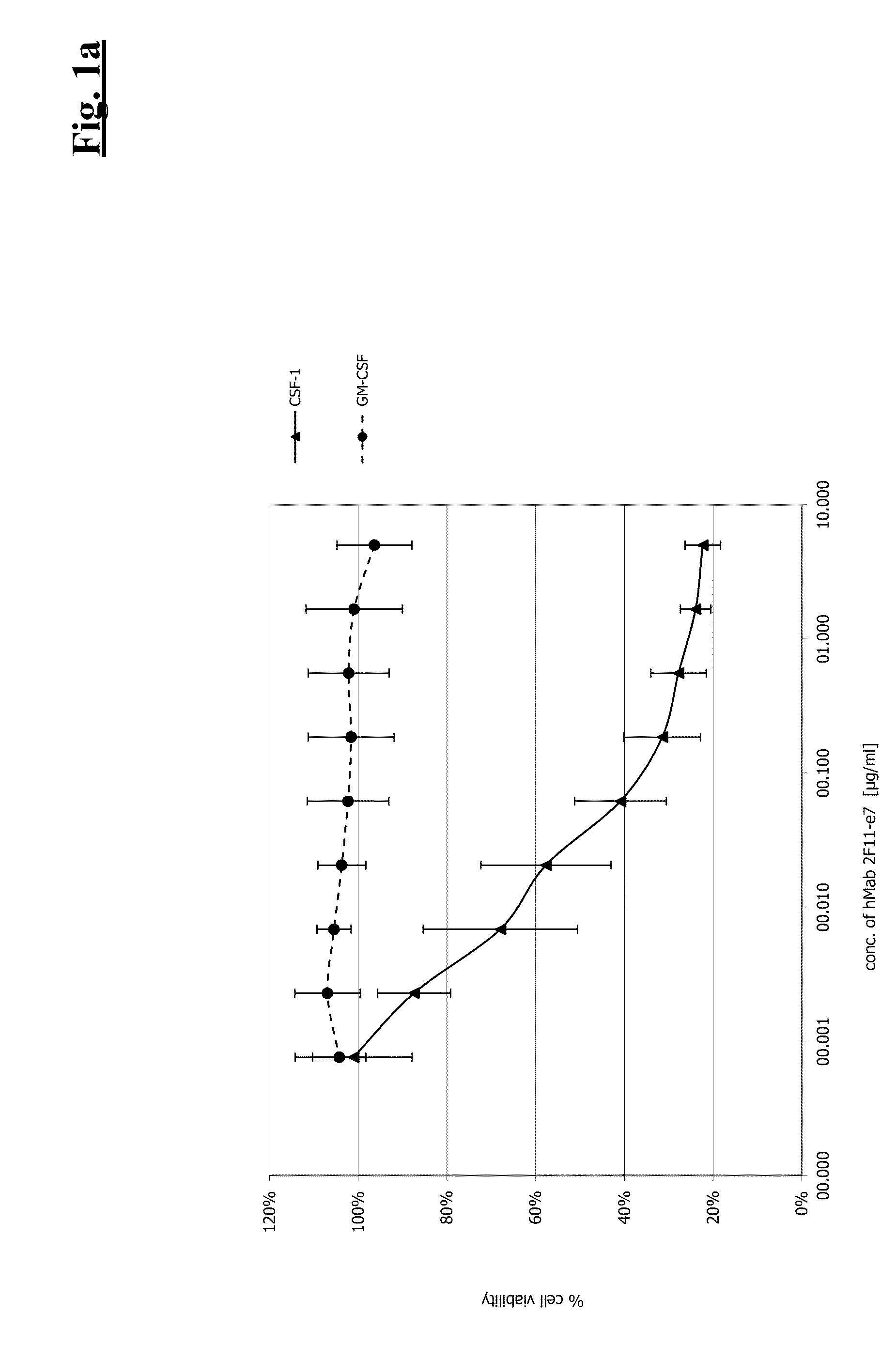
[0457]Human monocytes were isolated from peripheral blood using the RosetteSep™ Human Monocyte Enrichment Cocktail (StemCell Tech.—Cat. No. 15028). Enriched monocyte populations were seeded into 96 well microtiterplates (2.5×104 cells / well) in 100 μl RPMI 1640 (Gibco—Cat. No. 31870) supplemented with 10% FCS (GIBCO—Cat. No. 011-090014M), 4 mM L-glutamine (GIBCO—Cat. No. 25030) and 1× PenStrep (Roche Cat. No. 1 074 440) at 37° C. and 5% CO2 in a humidified atmosphere. When 150 ng / ml huCSF-1 was added to the medium, a clear differentiation into adherent macrophages could be observed. This differentiation could be inhibited by addition of anti-CSF-1R antibodies. Furthermore, the monocyte survival is affected and could be analyzed by CellTiterGlo (CTG) analysis. From the concentration dependent inhibition of the survival of monocytes by antibody treatment, an IC50 wa...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Immunogenicity | aaaaa | aaaaa |
| Light | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com