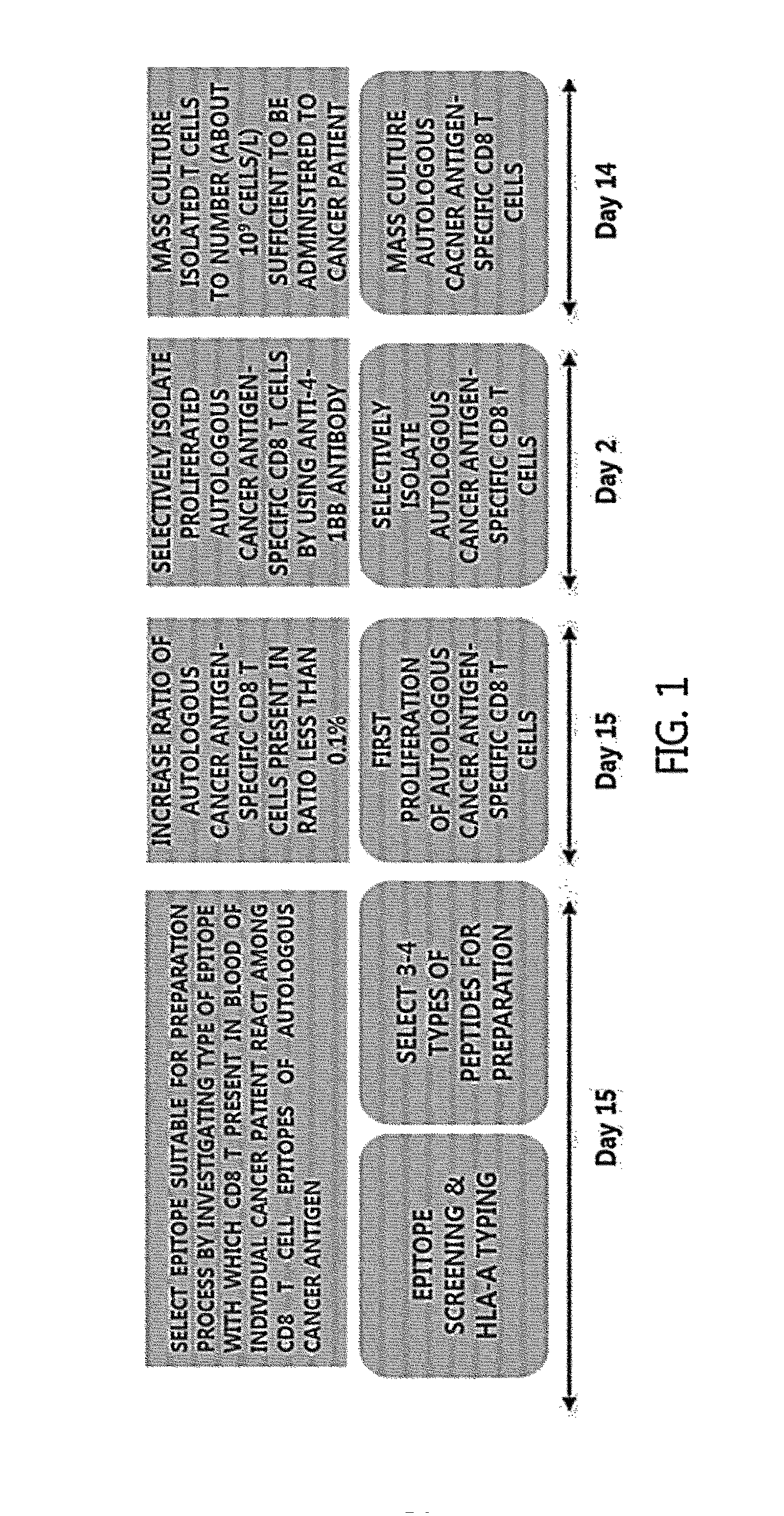
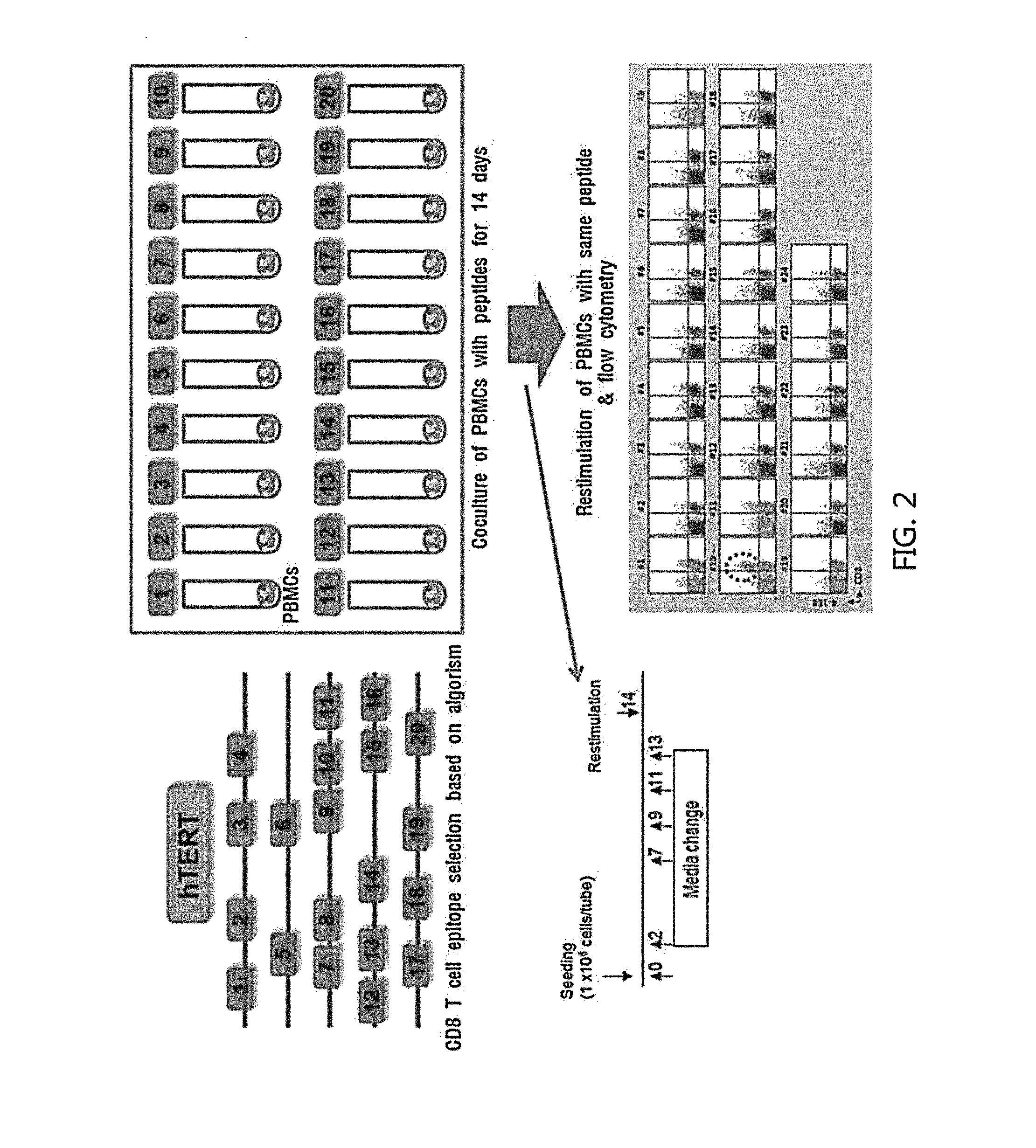
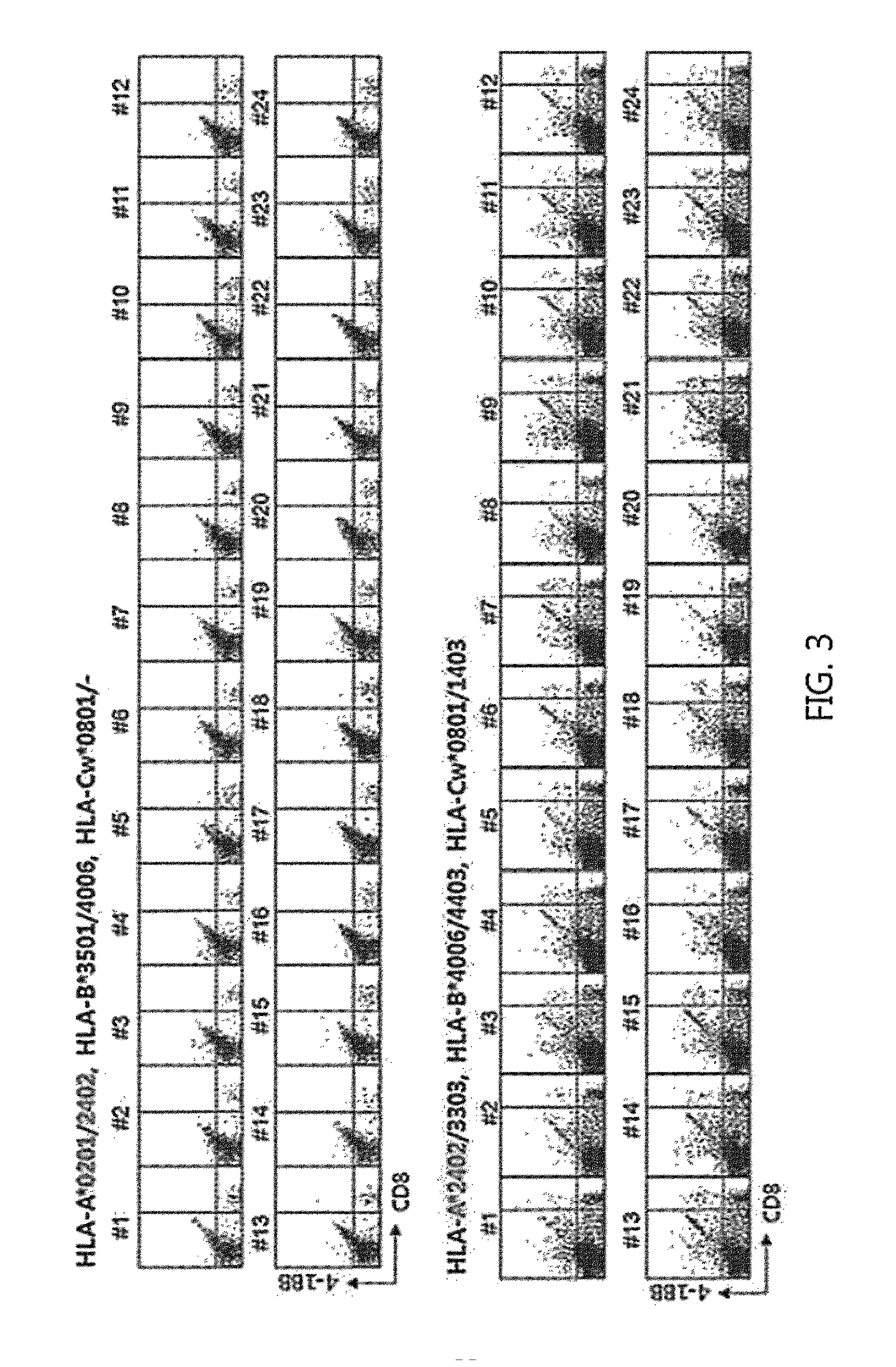
Methods for Isolating and Proliferating Autologous Cancer AntiGen-Specific CD8+ T Cells
a technology of autologous cancer and antigen-specific cd8, which is applied in the field of isolating and proliferating autologous cancer antigen-specific cd8 + t cells, can solve the problem of long culture period required to produce a sufficient amount of antigen-specific cd8, and achieve the effect of effectively selecting and eliminating cancer cells
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
experimental example
Epitope Screening Process
[0047]CD 8 T cell epitopes of an autologous cancer antigen were selected through algorithm. To evaluate a type of CD8 T cell epitope with which T cells present in blood of a cancer patient react, peripheral blood mononuclear cells (PBMCs) were isolated from blood of the cancer patient, washed, and suspended in CTL medium (RPMI1640 medium+4 mM L-glutamine+12.5 mM HEPES+50 μM 2-mercaptoethanol+3% autoplasma) to become the concentration of 1×106 cell / ml. Then, 1 ml of the suspension was aliquoted in a 14 ml round tube. Peptides for each epitope selected by the analysis with algorithm were added to each tube in the concentration of 1 μg / ml. Thereafter, culture in a CO2 incubator was started. Two days after culture, 1 ml of CTL medium including 50 U / ml IL-2 was added to each tube. On day 7, 9, 11, and 13 of culture, 1 ml of the medium was removed and CTL medium including 50 U / ml IL-2 was added. On day 14 of culture, RPMI1640 medium was added to each tube, and the...
example 1
Selection of Autologous Cancer Antigen and CD8 T Cell Epitope
[0049]Based on journals (Scanlan M J, et al., Immunol Rev. 2002 October 188:22-32; Ramakrishnan S, et al., Cancer research. 1998. 58:622-625; Nakahara Y, et al., Brain Tumor Pathol. 2004. 21(3):113-6) evaluating which type of cancer antigen is suitable for immunotherapy of a cancer depending on the type of cancer, an autologous cancer antigen, which is suitable for immunotherapy of frequently occurring cancer in Korean and hard-to treat cancers (e.g., gastric cancer, lung cancer and pancreatic cancer), is selected. hTERT (GenBank: BAC11010.1), WT1 (GenBank: AAO61088.1), NY-ESO1 (GenBank: CAA05908.1), and MAGE-A3 (NCBI Reference Sequence: NP—005353.1) are typical autologous cancer antigens used in anticancer immunotherapy in various ways, and types of cancers to which those four cancer antigens are applicable are selected and summarized in Table 1 below.
TABLE 1Target antigenPatientsEBVEBNA1, LMP1, LMP2EBV-related TumorsGast...
example 2
Epitope Screening on Clinical Cancer Patient
[0051]To evaluate whether CD8 T cell epitopes of autologous cancer antigens, i.e. hTERT, WT1, NY-ESO1, and MAGE-A3, selected in Example 1 substantially induce proliferation of CD8 T cells present in blood of a clinical cancer patient, epitope screening as depicted in FIG. 2. was performed. hTERT epitope screening was performed on gastric cancer, lung cancer and pancreatic cancer, as a main subject matter. Also, WT1 epitope screening was performed on brain and spinal cancer and lung cancer; NY-ESO1 epitope screening was performed on ovarian cancer and sarcoma; and MAGE-A3 epitope screening was performed on sarcoma and lung cancer as a main subject matter.
[0052]FIG. 3 shows an hTERT epitope screening result using PBMCs obtained from a healthy doner.
[0053]FIG. 4 shows a WT1 epitope screening result using PBMCs obtained from the healthy doner.
[0054]As shown in FIGS. 3 and 4, CD8 T cell epitopes of hTERT and WT1 did not induce T cell response b...
PUM
| Property | Measurement | Unit |
|---|---|---|
| concentration | aaaaa | aaaaa |
| concentration | aaaaa | aaaaa |
| concentration | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com