Method of treating c3 glomerulopathy
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
embodiments
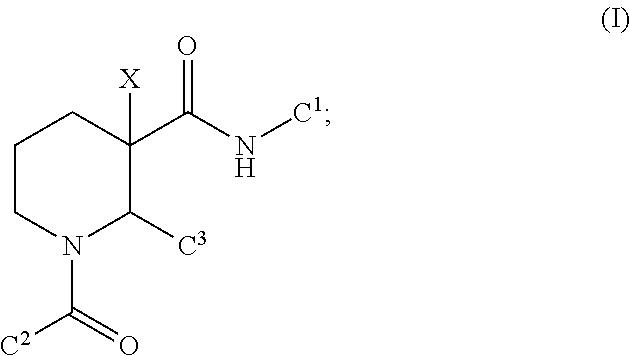
[0039]The present disclosure is directed to a method of treating a human suffering from or susceptible to complement 3 glomerulopathy comprising administering to the human an effective amount of a compound having the formula (I), or a pharmaceutically acceptable salt thereof,
wherein[0040]C1 is phenyl optionally substituted with from 1 to 3 R1 substituents;[0041]C2 is phenyl optionally substituted with from 1 to 3 R2 substituents;[0042]C3 is selected from the group consisting of C3-8 cycloalkyl and phenyl, and each C3 is optionally substituted with from 1-3 R3 substituents;[0043]each R1 is independently selected from the group consisting of[0044]halogen, —CN, —Rc, —CO2Ra, —CONRaRb, —C(O)Ra, —OC(O)NRaRb, —NRbC(O)Ra, —NRbC(O)2Rc, —NRaC(O)NRaRb, —NRaRb, —ORa, and —S(O)2NRaRb; wherein each Ra and Rb is independently selected from hydrogen, C1-8 alkyl, and C1-8 haloalkyl, or when attached to the same nitrogen atom can be combined with the nitrogen atom to form a five or six-membered ring ...
example 1
Compound 1 in a Patient with Progressive Complement 3 Glomerulonephritis
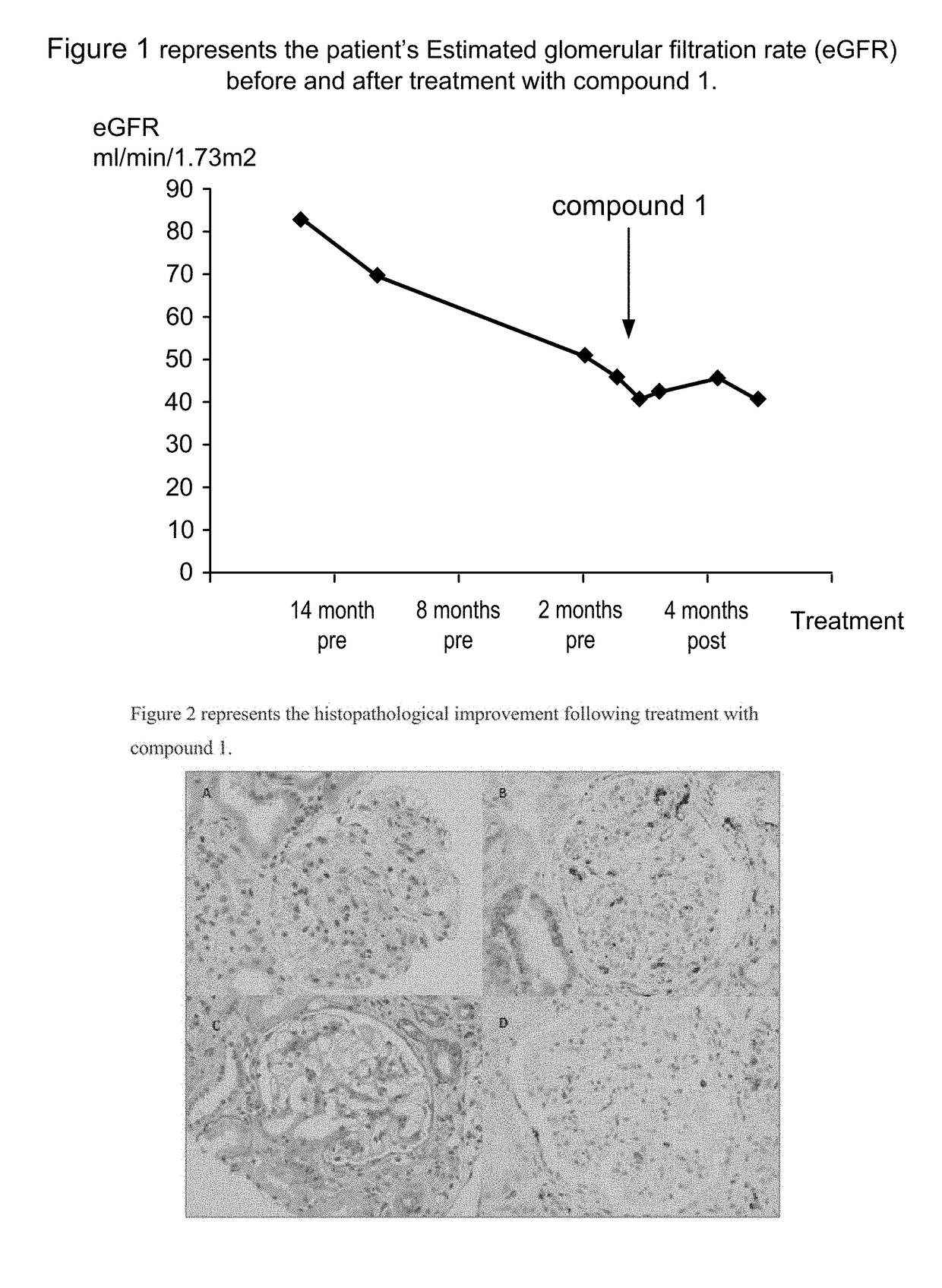
[0216]Under the Special Needs program in the United Kingdom (which is similar to a compassionate use protocol in the US), a C3 glomerulonephritis patient received treatment with the orally administered complement inhibitor compound 1, following the protocol detailed below. The patient had refractory disease despite a kidney transplant and prior treatment with the broadly immunosuppressive drugs rituximab, cyclophosphamide, mycophenolate mofetil, tacrolimus, and steroids. Renal allograft biopsies were taken pre-dose, 2 and 7 months during therapy.
Results:
[0217]The patient's condition improved in response to compound 1 treatment. The improvement seen with compound 1 treatment in this patient was based on the on-treatment kidney biopsy histologic findings that showed clearance ofglomerular endocapillary proliferation and a marked decrease in glomerular inflammatory macrophages compared to the pre-treatment biopsy. ...
example 2
zed, Double-Blind, Placebo-Controlled Phase 2 Study to Evaluate the Safety and Efficacy of Compound 1 in Patients with C3 Glomerulopathy
Protocol of the Study Planned
Aim
[0278]The aim of this study is to evaluate the effect of compound 1 treatment on renal disease activity in patients with complement 3 glomerulopathy (C3G). The intent is to slow down or improve renal disease with compound 1 treatment in these patients.
Objectives
[0279]The primary objective is to evaluate the efficacy of compound 1 compared to placebo based on histologic changes in C3G pathology from kidney biopsies taken before and during treatment.
[0280]The secondary objectives of this study include assessment of:[0281]1. The safety of compound 1 compared to placebo based on the incidence of adverse events, changes in clinical laboratory measurements, and vital signs;[0282]2. Changes in laboratory parameters of renal disease including estimated glomerular filtration rate (eGFR), proteinuria, and urinary excretion of m...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Time | aaaaa | aaaaa |
| Time | aaaaa | aaaaa |
| Time | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com