Treatment of autoimmune disorders with cd154 antibodies
a technology of cd154 and autoimmune disorders, applied in the field of autoimmune and inflammatory diseases, can solve the problems of inadequate therapeutic armamentarium, discontinued hu5c8 development, and large number of side effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
[0095]Determination of Binding Affinity.
[0096]Biamolecular Interaction Analysis (BIA) was performed using a BIAcore 3000 instrument. Affinipure goat F(ab′)2 fragment specific for human IgG, F(ab′)2 fragment was immobilised on a CM5 Sensor Chip via amine coupling chemistry to a capture level of approximately 4000 response units (RUs). HBS-EP buffer (10 mM HEPES pH 7.4, 0.15 M NaCl, 3 mM EDTA, 0.005% (v / v)) Surfactant P20) was used as the running buffer with a flow rate of 10 μL / minute (min). A 10 μL injection of test CD154 antibody or Fab at 10 μg / mL was used for capture by the immobilised anti-human IgG-F(ab′)2. Human CD154 was titrated over the captured CD154 antibody or Fab at various concentrations (1 nM or below) at a flow rate of 3 μL / min. The surface was regenerated by 2×10 μL injections of 40 mM HCl, followed by a 5 μL injection of 5 mM NaOH at a flow rate of 10 μL / min.
example 2
[0097]Study SL0013:
[0098]A clinical study of two parts in which, during the first part, ascending single intravenous doses of CDP7657 were assessed for safety, tolerability and pharmacokinetic profiles at 0.004 mg / kg, 0.02 mg / kg, 0.1 mg / kg, 0.5 mg / kg and 1.7 mg / kg in 5 groups, each of 3 healthy male volunteers, followed by a dose of 5 mg / kg in two more groups of 3 healthy male volunteers and 3 healthy female volunteers. Following establishment of acceptable profiles, the second part of the study assessed single intravenous doses at 5 mg / kg, 15 mg / kg, 30 mg / kg and 60 mg / kg in 4 groups of 3 patient volunteers (who had an established diagnosis of systemic lupus erythematosus; SLE). In addition to further assessments of safety, tolerability and pharmacokinetics, the pharmacodynamic effects were also explored using various disease markers.
[0099]In performing this study, the basic characteristics of both the anti-CD154 antibody / antibody fragment and its PEG component were established.
example 3
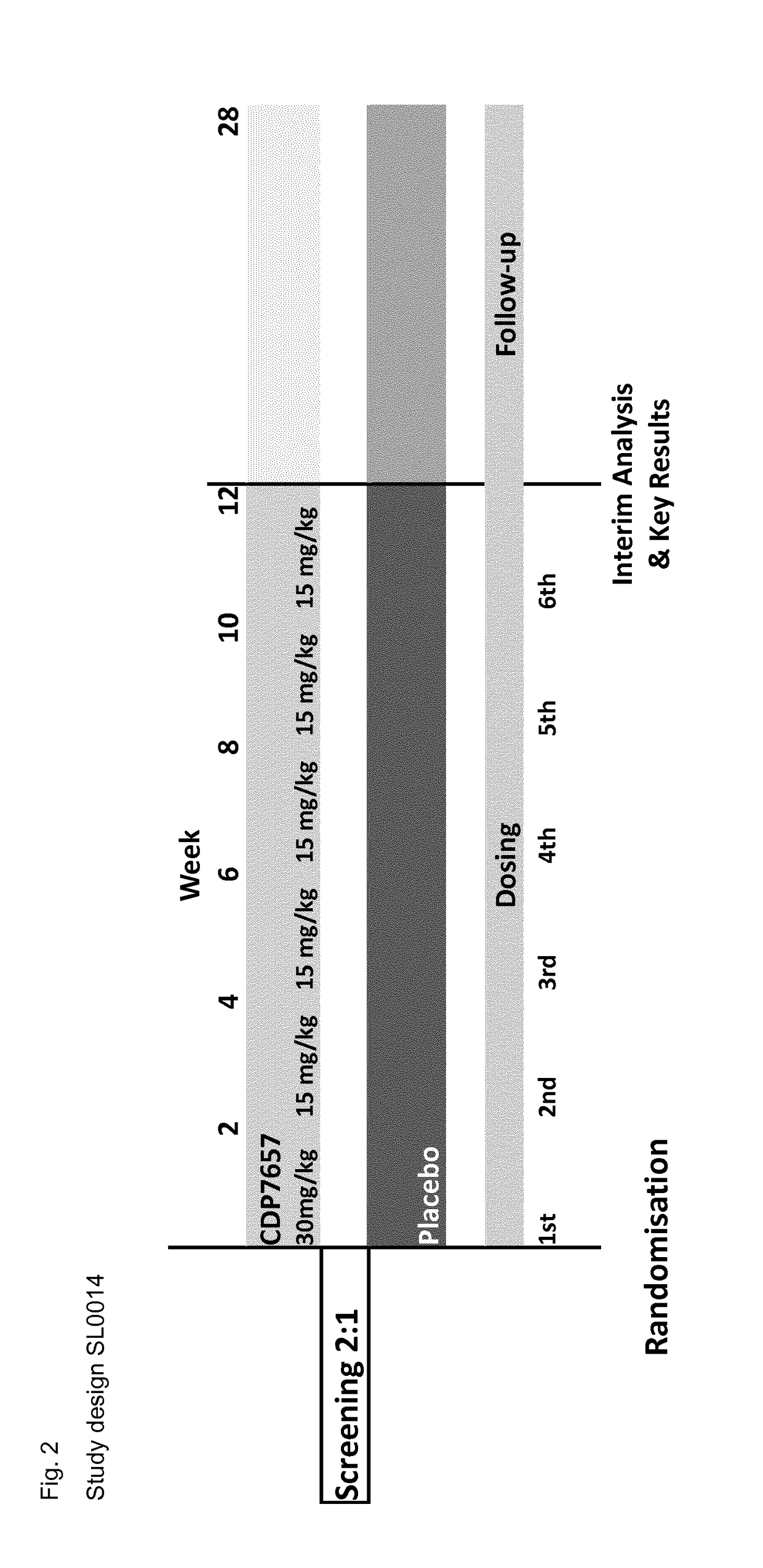
[0100]Study SL0014:
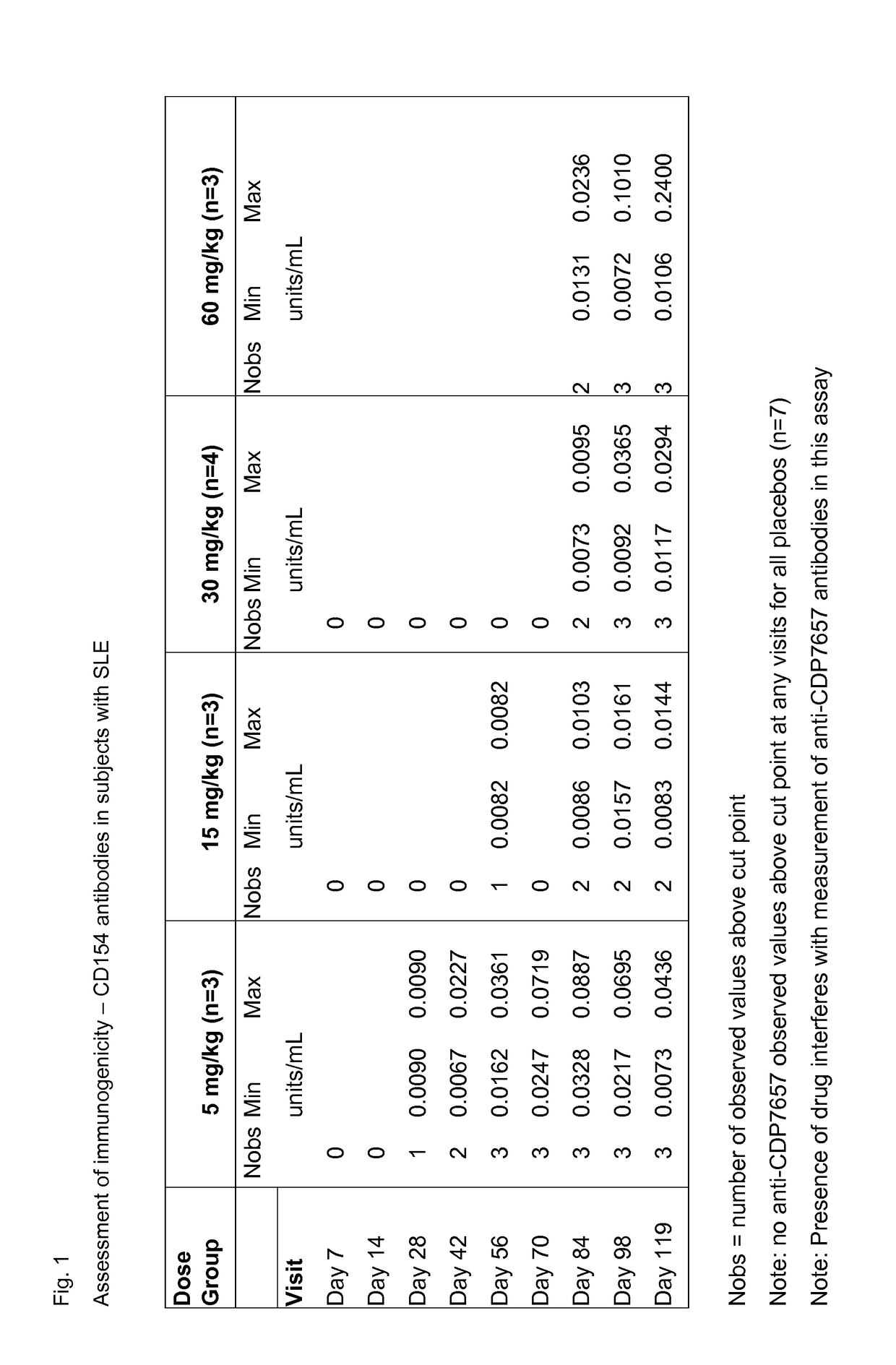
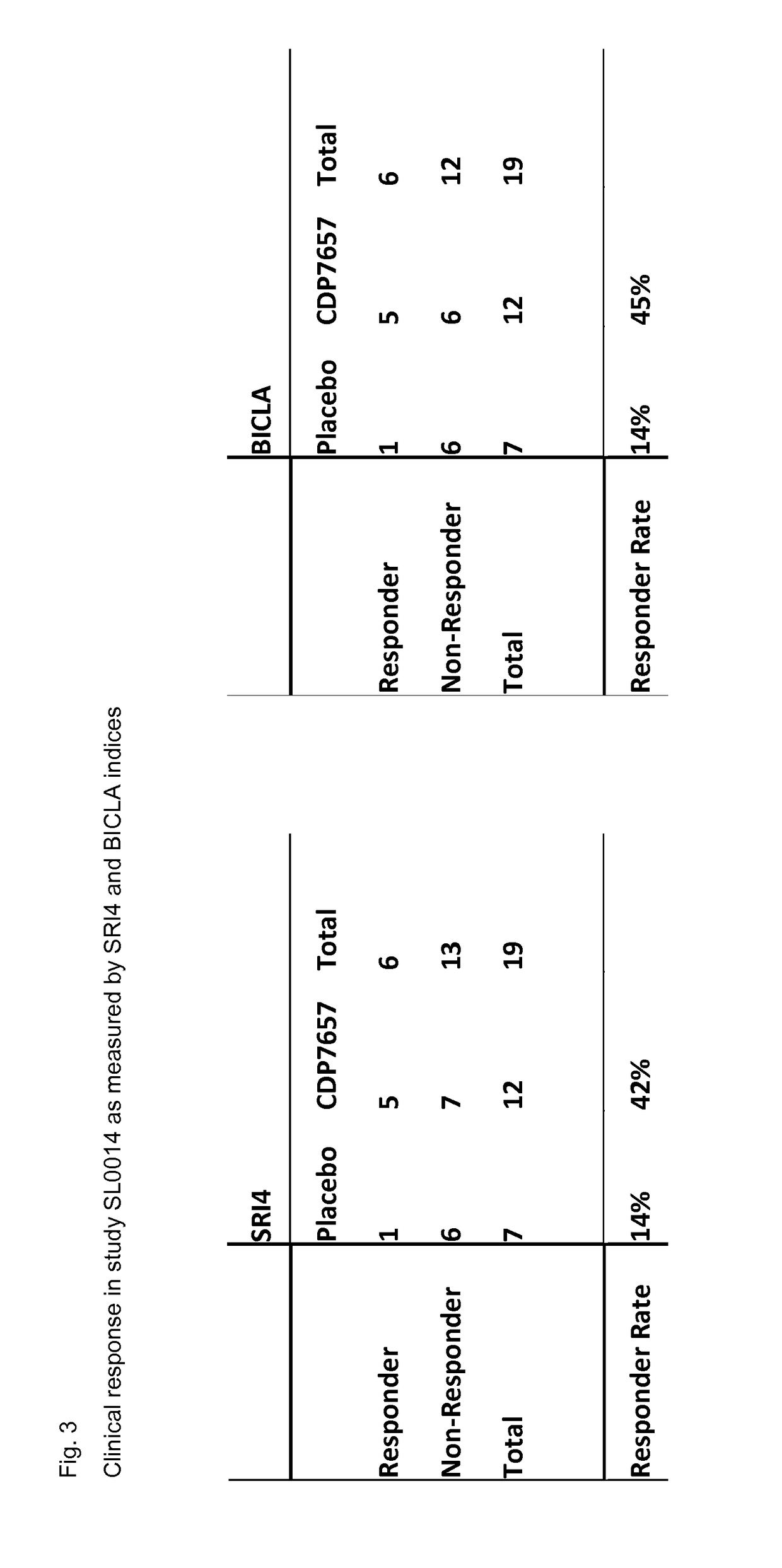
[0101]A clinical study in which patients with an established diagnosis of SLE were randomised in a double-blind fashion to receive six intravenous doses of CDP7657 (n=16) or matching placebo (n=8) over a period of 10 weeks. The dosing regimen under test (in those receiving active drug) comprised a single loading dose of 30 mg / kg followed by a maintenance dose of 15 mg / kg every 2 weeks thereafter, for a total of 6 doses of CDP7657. In addition to assessing the safety, tolerability and pharmacokinetic profiles of CDP7657 versus placebo, the study explored the immunogenicity (both anti-CD154 and PEG components), effects on various disease markers and effects on clinical disease parameters during and for 18 weeks after treatment. In this way the safety, tolerability and ability of the dosing regimen to deliver disease-modifying effects were established.
PUM
| Property | Measurement | Unit |
|---|---|---|
| flow rate | aaaaa | aaaaa |
| flow rate | aaaaa | aaaaa |
| frequency | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com