Methods of treatment
a treatment method and technology of posaconazole, applied in the field of treatment methods, can solve the problems of serious and potentially life-threatening side effects, posaconazole treatment, and the inability to safely administer cyp3a4 substrate drugs immediately after a patient, so as to avoid or reduce the incidence of side effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
inetic Studies with Posaconazole and Lurasidone
[0408]Inventors studied 6 obese male and female subjects (ages 18-50, BMI>35) taking Posaconazole oral tablets (300 mg qd) and Lurasidone (20 mg qd). Body weights and BMI measurements for the 6 subjects are provided below in Table 1.
TABLE 1Subject DemographicsSubject #Weight (kg)BMI (kg / m2)101-001111.845101-002136.844.4101-005137.751.2101-007103.736.8101-008122.339.8101-010120.043.9
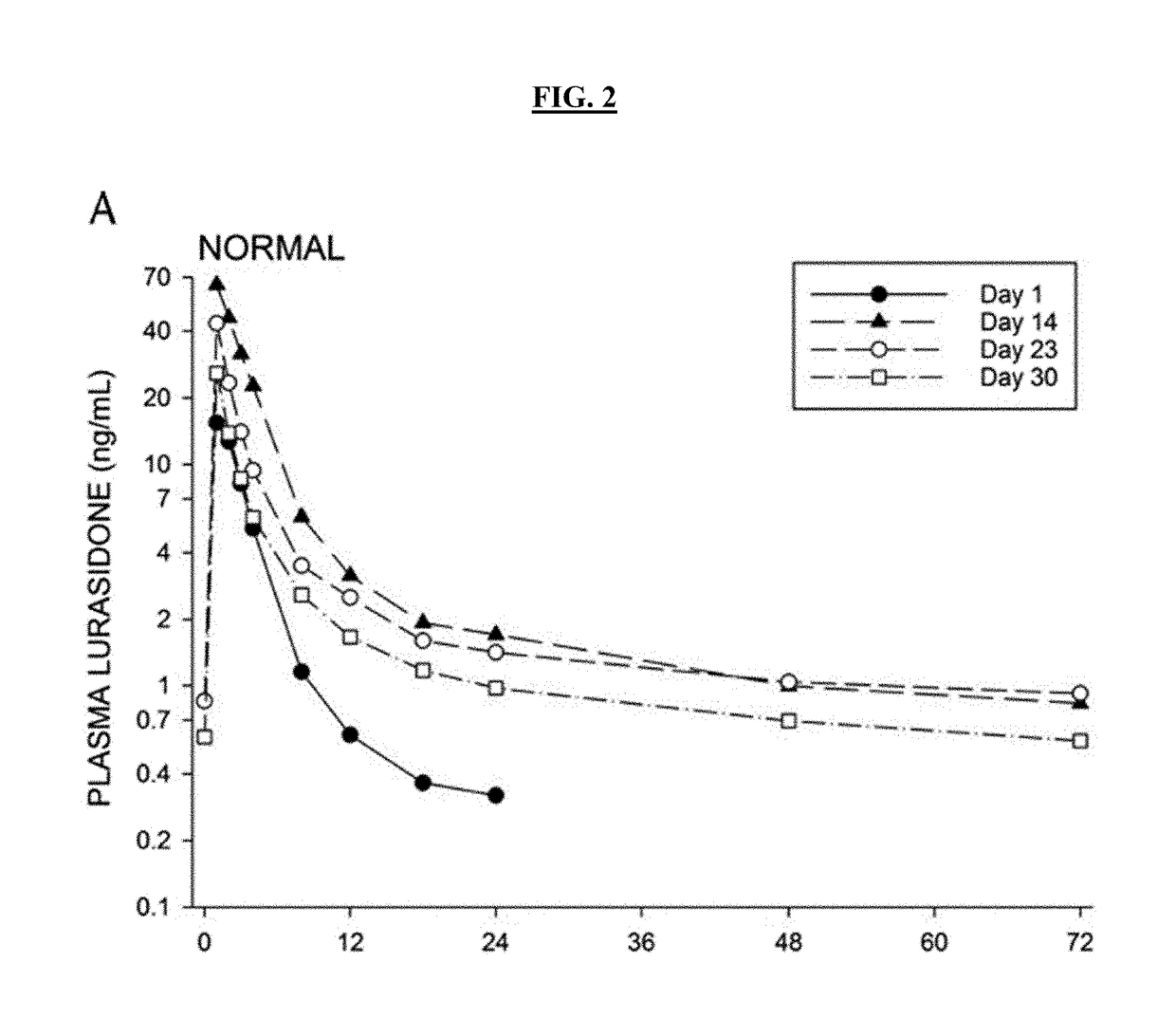
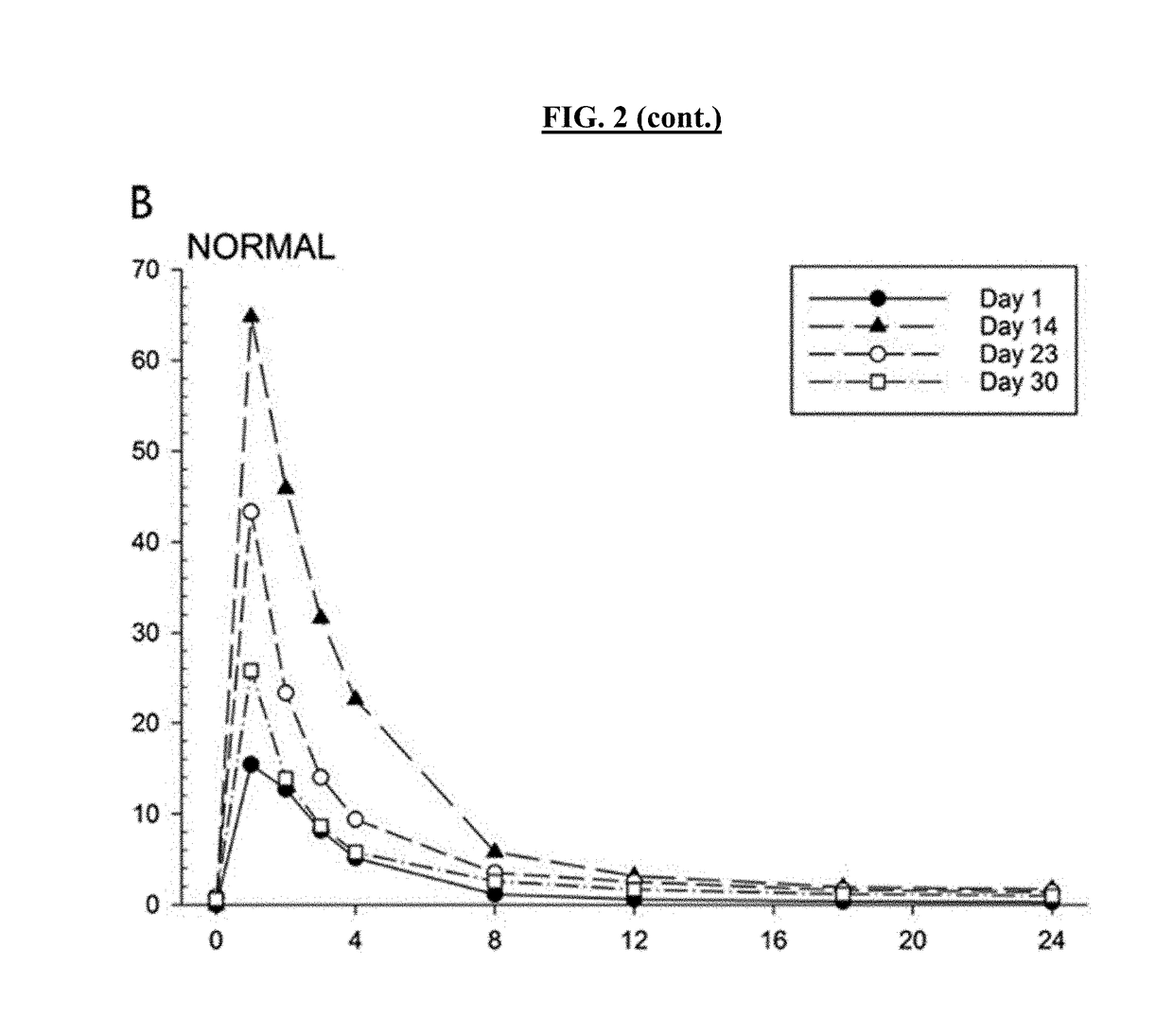
[0409]Subjects were dosed with Lurasidone alone on Day 1, then subsequently dosed to steady-state Posaconazole levels, with a loading dose of 300 mg twice a day on Day 2 and 300 mg once a day thereafter over a period of 14 days. Posaconazole administration was then stopped and Lurasidone (20 mg qd) administered 2, 4, and 6 days after administration had ceased (studies days 17, 19, and 21 respectively). Lurasidone AUC was measured for 24 hours after each administration. Table 2 shows subject Lurasidone AUC levels 2, 4 and 6 days after Posaconazole was stopped,...
example 2
Impairment of Lurasidone Clearance after Discontinuation of Posaconazole. Impact of Obesity, and Implications for Patient Safety
[0415]The following studies were reported by Greenblatt et al., J. Clin. Psychopharmacol., 2018; 38(4):289-295 (doi: 10.1097 / JCP.0000000000000892), which is herein incorporated by reference in its entirety for all purposes.
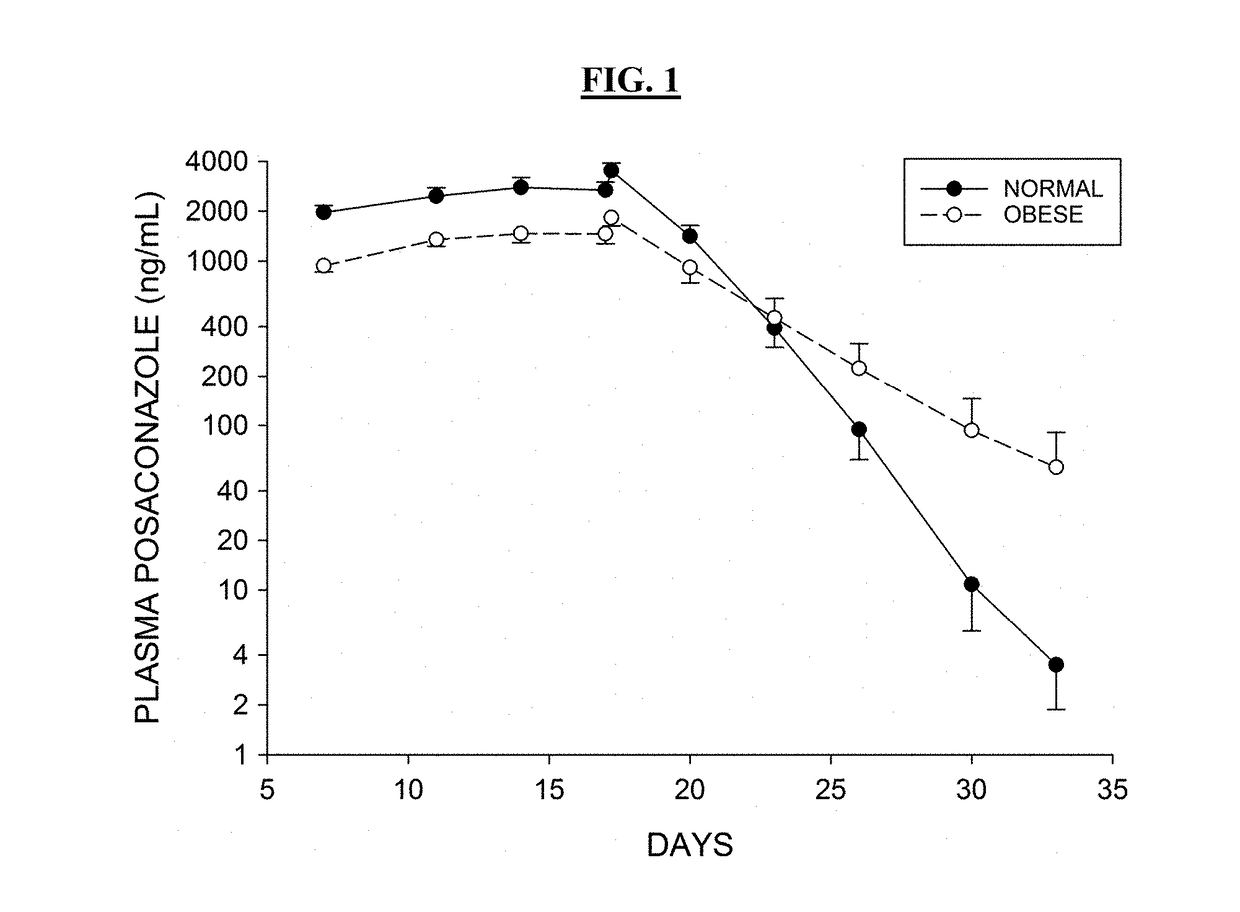
[0416]The antipsychotic agent lurasidone is metabolized by Cytochrome P450-3A (CYP3A) enzymes. Coadministration with strong CYP3A inhibitors (such as ketoconazole, posaconazole, and ritonavir) is contraindicated due to the risk of sedation and movement disorders from high levels of lurasidone. This study evaluated the time-course of recovery from the posaconazole drug interaction, and the effect of obesity on the recovery process.
[0417]With posaconazole coadministration, lurasidone area under the concentration curve (AUC) increased by an arithmetic mean factor of 6.2 in normals, and by 4.9 in obese subjects. Post-treatment washout of posa...
example 3
ce of a Posaconazole-Mediated Drug-Drug Interaction with Ranolazine after Cessation of Posaconazole Administration: Impact of Obesity and Implications for Patient Safety
[0445]The following studies were reported by Chow et al., J. Clin. Pharmacology. 2018; 0(0):1-7 (doi: 10.1002 / jcph.1257), which is herein incorporated by reference in its entirety for all purposes.
[0446]The antianginal agent ranolazine is metabolized primarily by cytochrome P450-3A (CYP3A) enzymes. Coadministration with strong CYP3Ainhibitors, such as ketoconazole and posaconazole, is contraindicated due to risk of QT prolongation from high levels of ranolazine. This study evaluated the time course of recovery from the posaconazole drug interaction in normal-weight and otherwise healthy obese subjects. Subjects received single doses of ranolazine in the baseline control condition, again during coadministration of posaconazole, and at 4 additional time points during the 2 weeks after posaconazole discontinuation. With...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Weight | aaaaa | aaaaa |
| Length | aaaaa | aaaaa |
| Fraction | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com