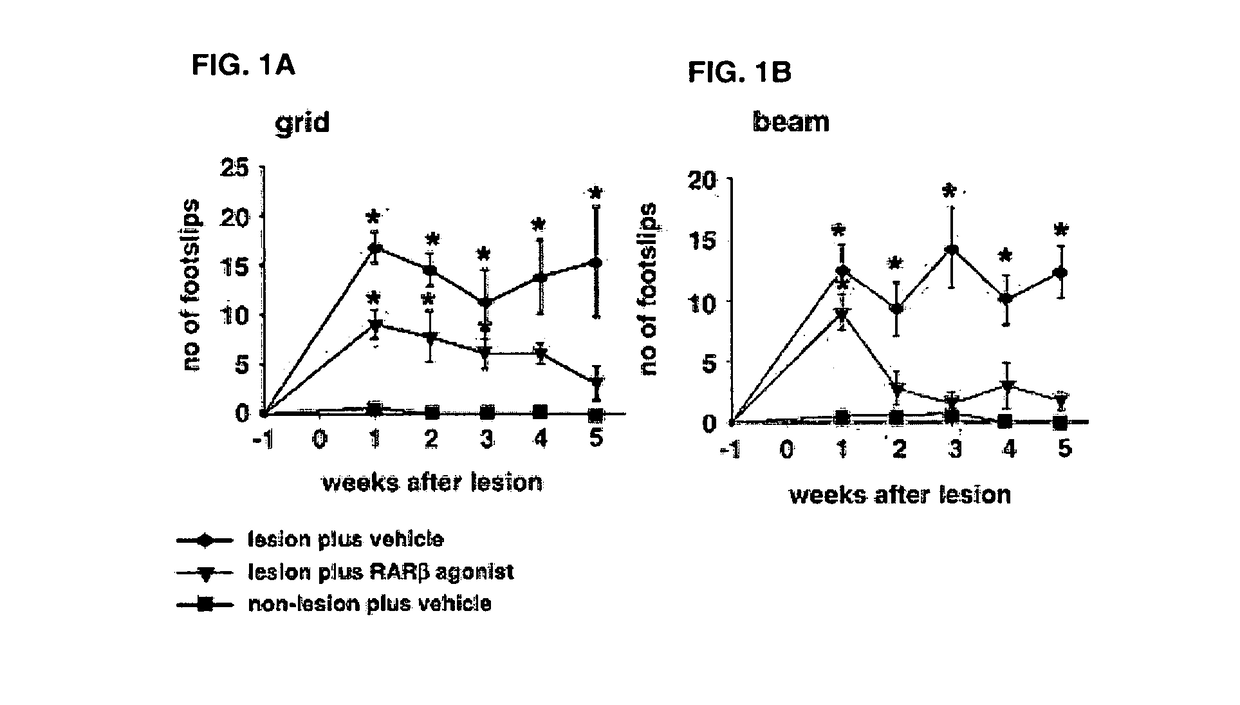
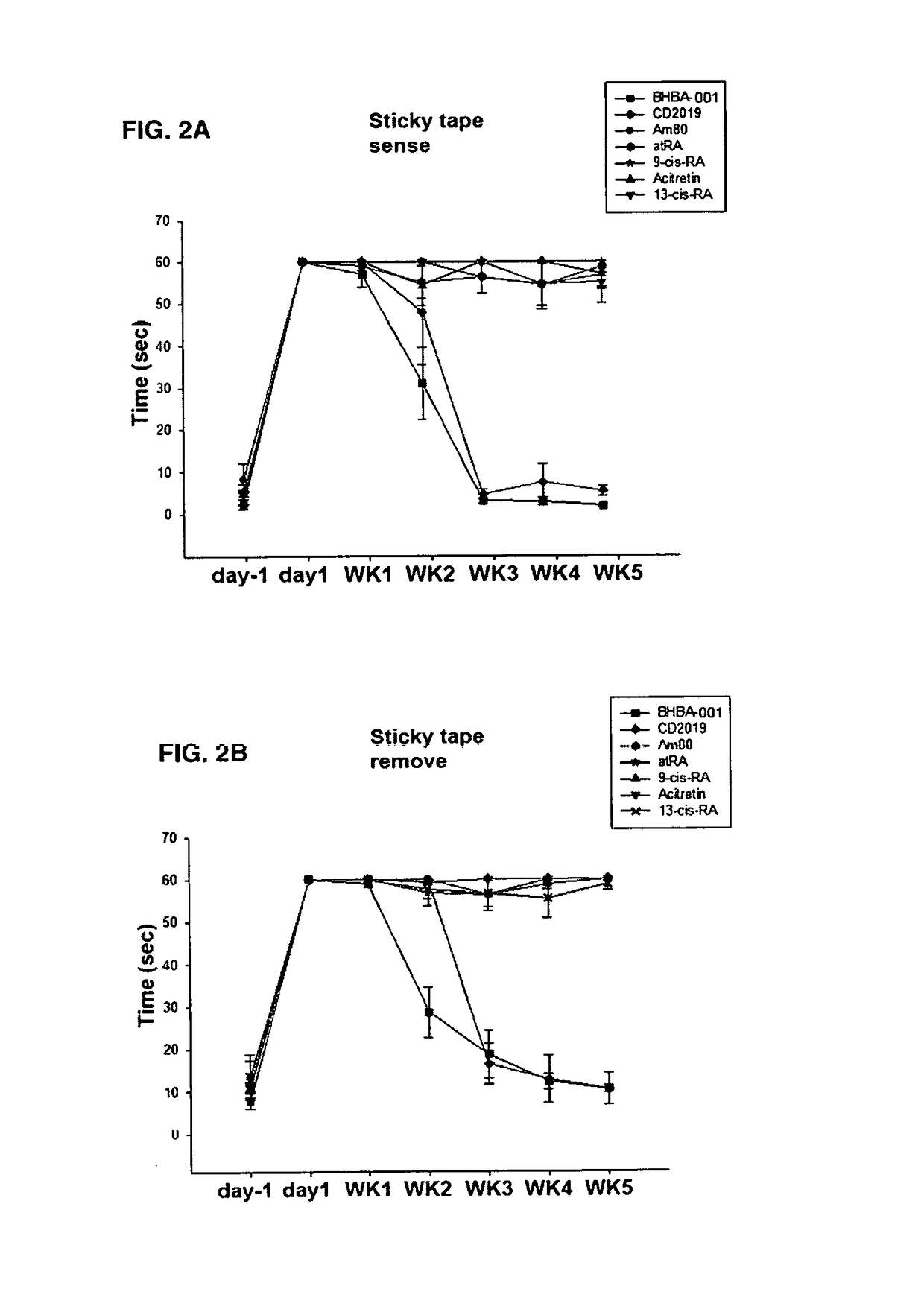
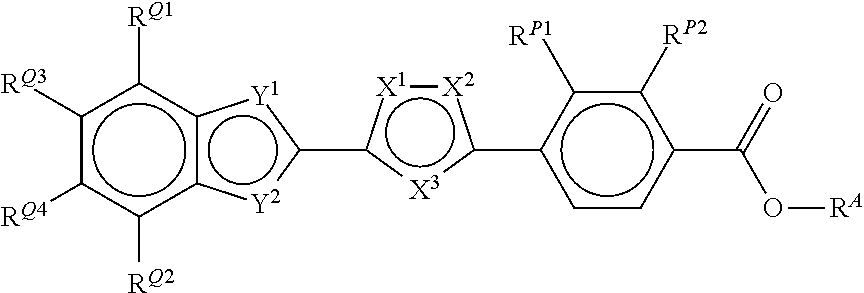
Bicycloheteroaryl-Heteroaryl-Benzoic Acid Compounds as Retinoic Acid Receptor Beta (RARBeta) Agonists
a technology agonist, which is applied in the field of therapeutic compounds, can solve the problems of lack of appropriate ‘growth programme’ of damaged neurones, inability to effectively treat nerve injuries, and inability to achieve the effect of enhancing the growth rate of retinoic acid receptors
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
examples
[0468]The following examples are provided solely to illustrate the present invention and are not intended to limit the scope of the invention, as described herein.
Abbreviations
[0469]Ac=acetyl
aq.=aqueous
Boc=tert-butoxycarbonyl
br=broad
Bu=butyl
conc.=concentrated
CDl=1,1-carbonyldiimidazole
d=doublet
DCM=dichloromethane
DIPEA=N,N-diisopropylethylamine
[0470]DMAP=4-dimethylaminopyridine
DMF=N,N-dimethylformamide
[0471]DMSO=dimethylsulfoxide
DPPF=1,1′-bis(diphenylphosphino)ferrocene
[0472]EDC=1-Ethyl-3-(3-dimethylaminopropyl)carbodiimide hydrochloride
eq.=no. of molar equivalents
ES=electrospray
Et=ethyl
h=hour(s)
HATU=2-(7-aza-1H-benzotriazole-1-yl)-1,1,3,3-tetramethyluronium hexafluorophosphate
HOBt=N-hydroxybenzotriazole
[0473]HPLC=high performance liquid chromatography
Hz=hertz
L=litre
M=molar
m=multiplet
m-CPBA=meta-chloroperoxybenzoic acid
Me=methyl
min=minute(s)
NBS=N-bromosuccimide
[0474]NMR=nuclear magnetic resonance
Ph=phenyl
PPA=polyphosphoric acid / pyrop...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle size | aaaaa | aaaaa |
| concentration | aaaaa | aaaaa |
| concentration | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com