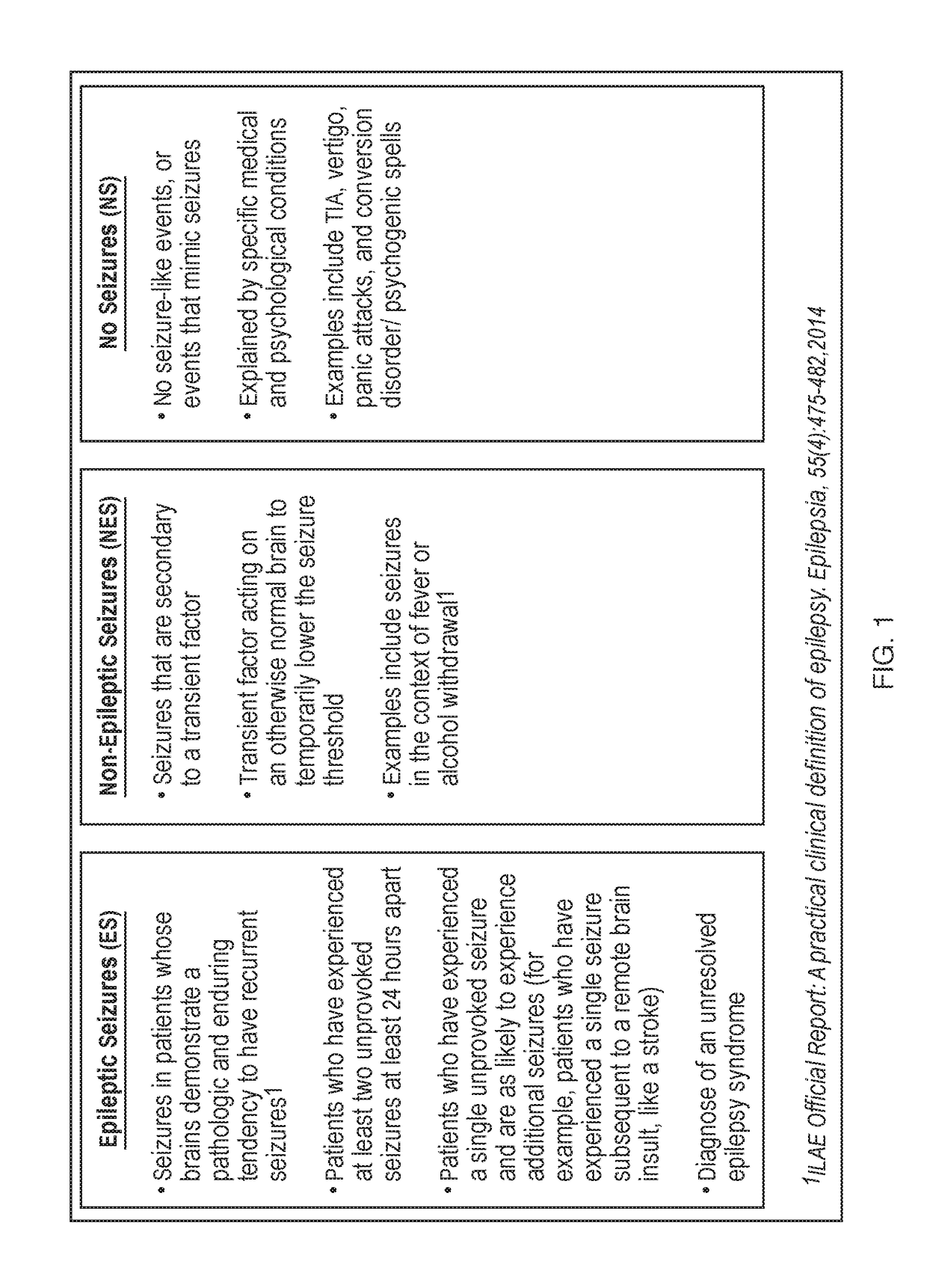
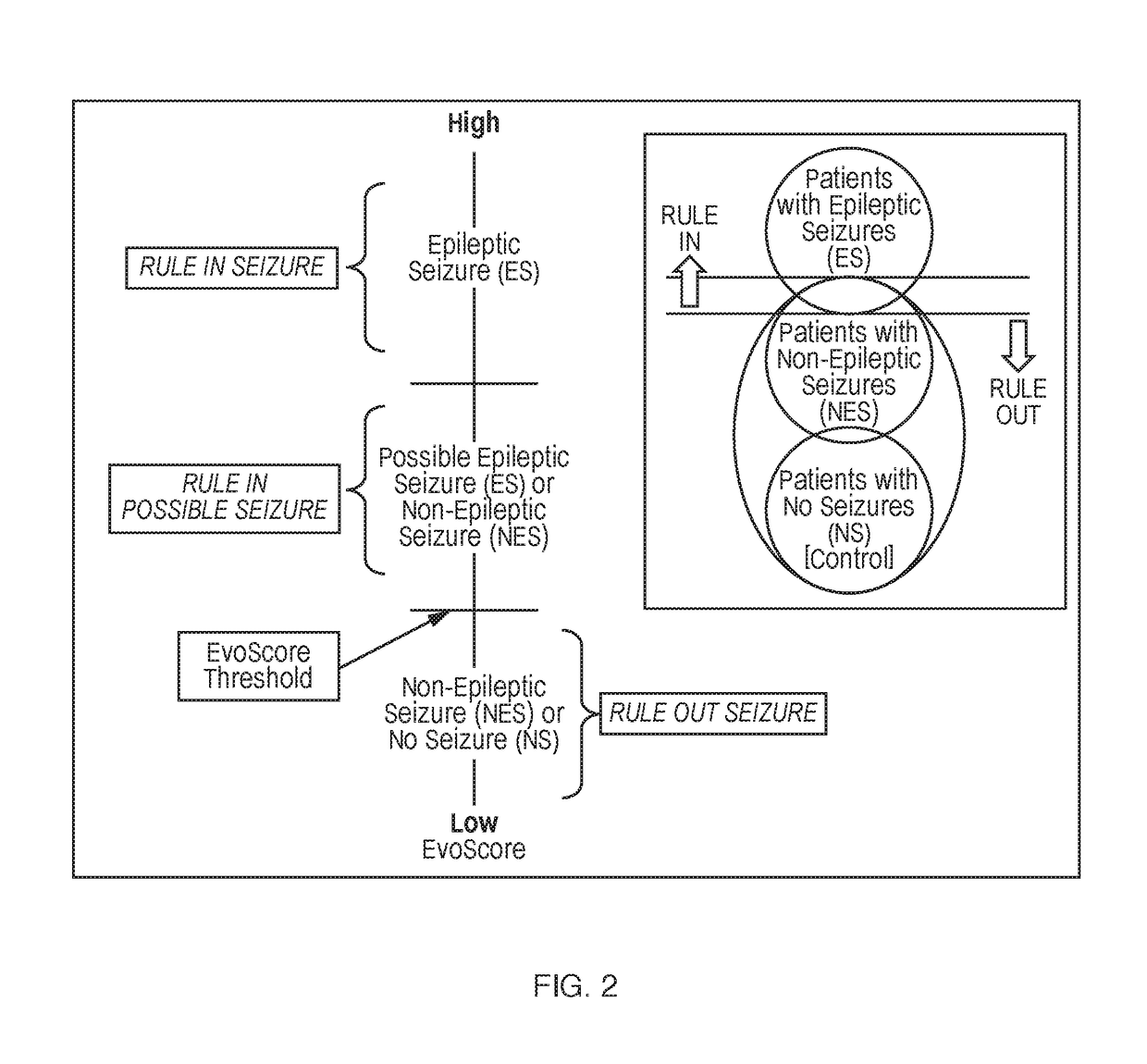
Biomarkers and methods for detection of seizures and epilepsy
a technology for epilepsy and biomarkers, applied in the field of biomarkers and methods for detection of seizures and epilepsy, can solve the problems of significant financial burden, significant morbidity, health care cost, even mortality, and significant financial burden of epilepsy care, and achieve the effect of monitoring patients over time and strong diagnostic performan
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
emographics and Biomarker Data: Inpatients, Outpatients and Normal Controls
[0171]Inpatient, outpatient and normal controls are shown in Table 1. Biomarkers alone including TARC and sICAM5, and the ratio of biomarkers TNFα / TARC and TNFα / sICAM5 were demonstrated to have statistically significant differences (p<0.05) between Normal Controls and Event Diagnosis, between Normal Controls and Patient Diagnosis 1, and between Normal Controls and Patient Diagnosis 1 & 2. These biomarkers and ratios of biomarkers can be used alone or in combination for the determination of seizure or not and epilepsy or not.
TABLE 1Patient Demographics and CharacteristicsPatientPatientEventNormalDiagnosis 1Diagnosis 1 & 2DiagnosisControlsVariable(N = 83)(N = 99)(N = 28)(N = 29)Age45.345.347.445.618-3019.3%19.2%10.7%20.7%31-4019.3%20.2%17.9%10.3%41-5030.1%28.3%35.7%20.7%51+31.3%32.3%35.7%48.3%SexMale27.7%28.3%17.9%31.0%Female72.3%71.7%82.1%69.0%LabsTNFalpha79.779.783.280.0TARC636.9635.7649.2511.9SlCAM518,236.01...
example 2
gnosis 24 Hours by Logistic Regression
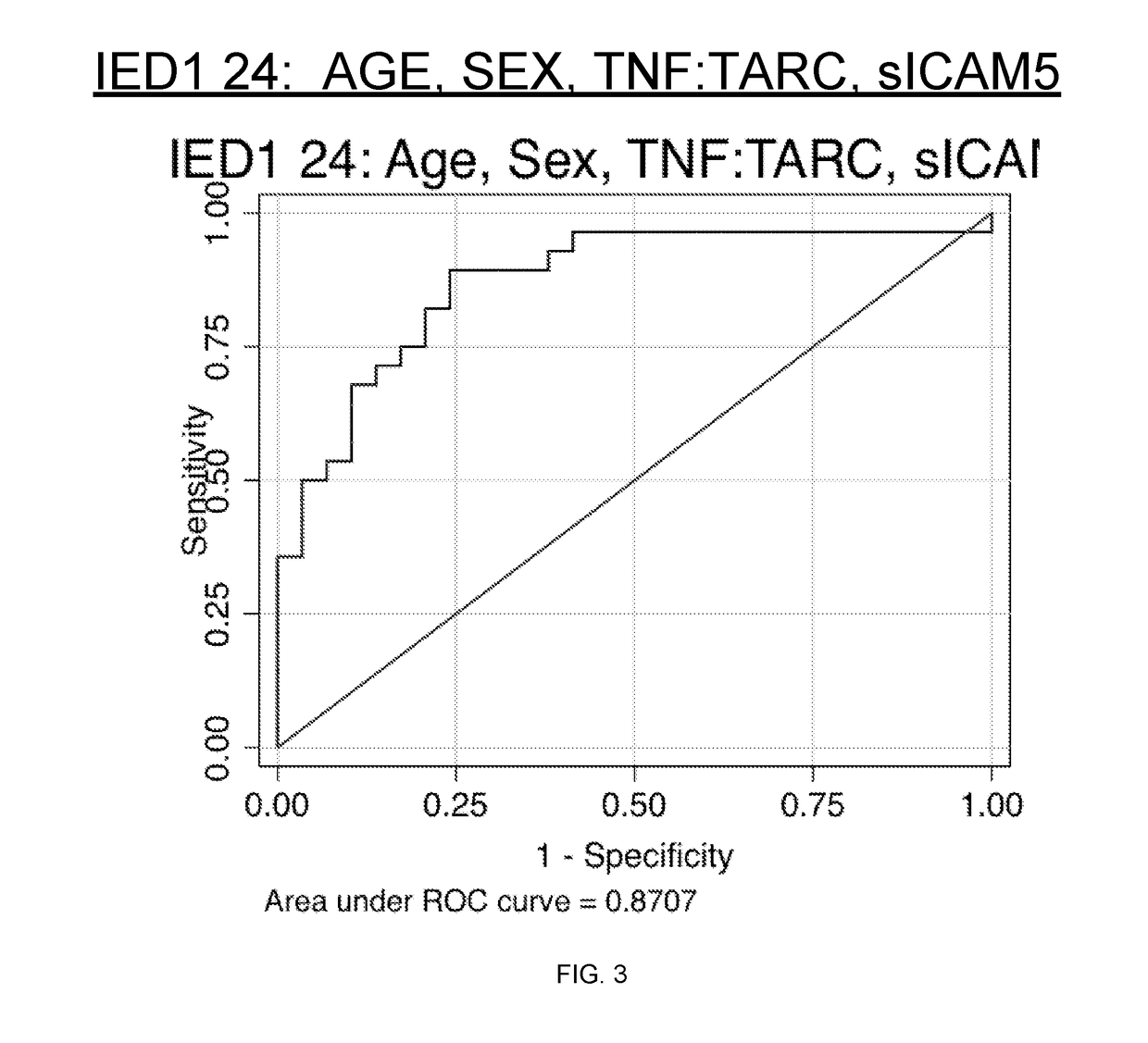
[0172]Logistic Regression Model results may be used to classify events as either seizure / epileptic or no event. The data contains samples collected within 24 hours of an event. EvoScore algorithms were determined to be a function of measurable changes of the concentration for TARC, sICAM5 and TNF-α and patient physical characteristics, including age and sex.
[0173]EvoScore demonstrated a Receiver Operating Characteristic (ROC) AUC of 0.8707 with 95% confidence interval of 0.7739 to 0.9675, Diagnostic Sensitivity of 89.3% (designed to maximize), Specificity of 75.9%, Positive Predictive Value of 78.1%, Negative Predictive Value of 88% and Accuracy of 82.5% (designed to maximize) for Patients with blood drawn within 24 hours of event when comparing patients with phasic and measureable changes for seizures versus normal controls. The results are summarized in TABLE 2 and FIG. 3.
example 3
tudies: Event Diagnosis within 72 Hours by Logistic Regression
[0174]Multivariate Logistic Regression Model results may be used classify events as either seizure / epileptic or no event. The data contains samples collected within 72 hours of an event. EvoScore algorithms were determined to be a function of measurable changes of the concentration for TARC, sICAM5 and TNF-α and patient physical characteristics, including age and sex.
[0175]EvoScore demonstrated a ROC AUC of 0.8452 with 95% confidence interval of 0.7552 to 0.9353, Diagnostic Sensitivity of 84.4% (designed to maximize), Specificity of 72.4%, Positive Predictive Value of 82.6%, Negative Predictive Value of 75% and Accuracy of 79.7% (designed to maximize) for Patients with blood drawn within 72 hours of event when comparing patients with phasic and measureable changes for seizures versus normal controls. Results are summarized in Table 3.
TABLE 3Results of event diagnosis (24 hours) by Logistic Regression.95% ConfidenceVariabl...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Fraction | aaaaa | aaaaa |
| Fraction | aaaaa | aaaaa |
| Fraction | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com