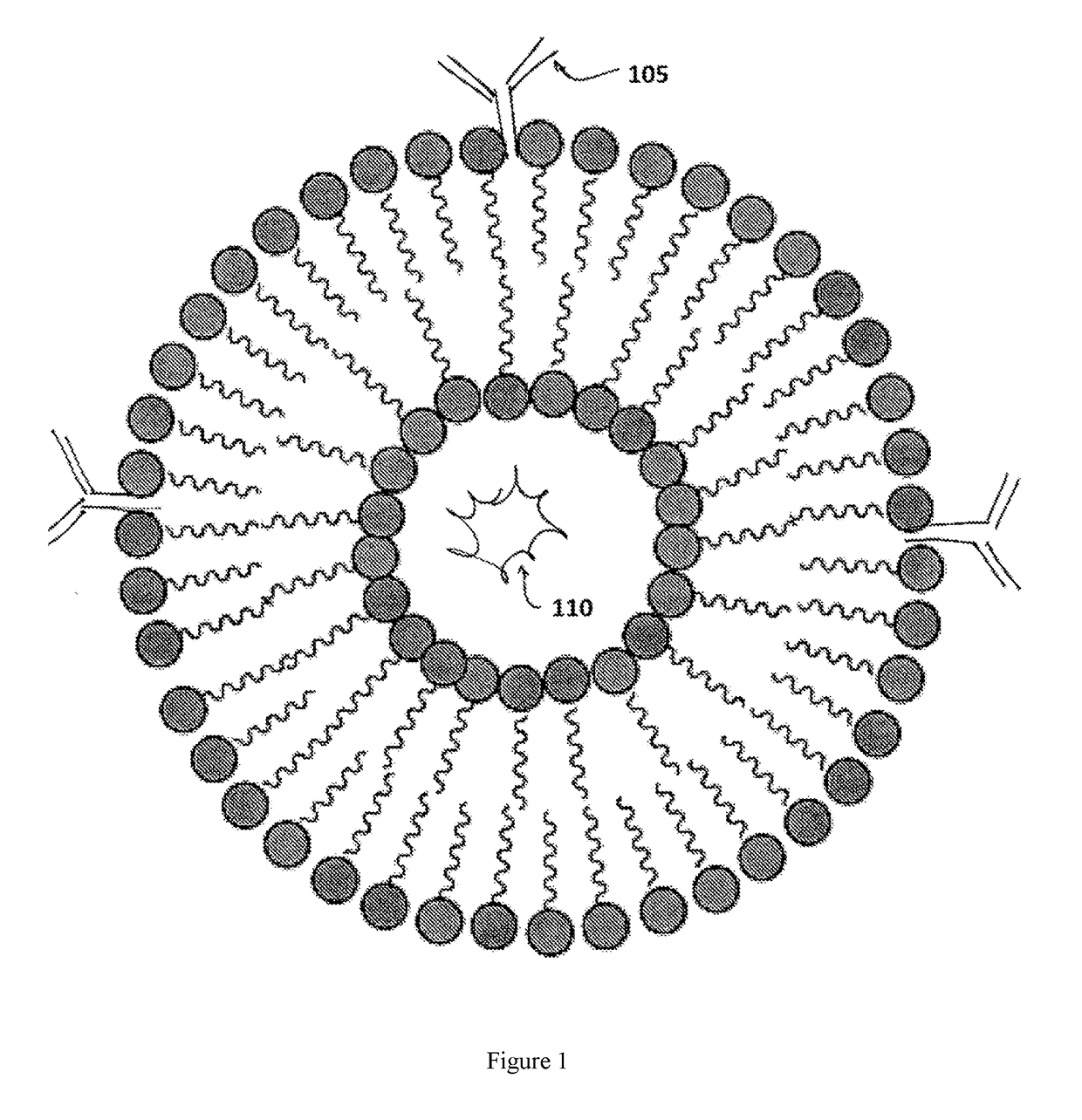
A sterically stabilized carrier for subcutaneous, sublingual, and oral therapeutics, compositions and methods for treating a mammal
a carrier and sterically stabilized technology, applied in the direction of antibacterial agents, drug compositions, immunological disorders, etc., can solve the problems of poor clinical efficacy, poor treatment effect, and relatively short life of the mammal body, and achieve the effects of improving the therapeutic effect of food allergy, improving the therapeutic effect, and improving the therapeutic
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
and Methods
[0149]Animals
[0150]Six week-old male C57 black 6 mice were purchased from Charles River Laboratories, Inc., Wilmington, Mass. The animals were provided with an ovalbumin-free diet and water ad libitum and were housed in an environment-controlled, pathogen-free animal facility. All animal protocols were approved by the Animal Care Committee of the University of Illinois at Chicago, the Medical College of Wisconsin and the Zablocki Veterans Administration Medical Center, and were in agreement with the National Institute of Health's guidelines for the care and use of laboratory animals.
[0151]Ovalbumin Sensitization
[0152]The animals were sensitized with ovalbumin (OVA). On day 0, each mouse was anesthetized with methoxyflurane given by inhalation. A fragmented heat-coagulated OVA implant was inserted subcutaneously on the dorsal aspect of the cervical region.
[0153]For a ten-day period (days 14-24), each mouse was given a 30-minute aerosolization of a 6% OVA solution on altern...
example 2
mparison of BUD in the Carrier with Conventional Liposomes
[0178]
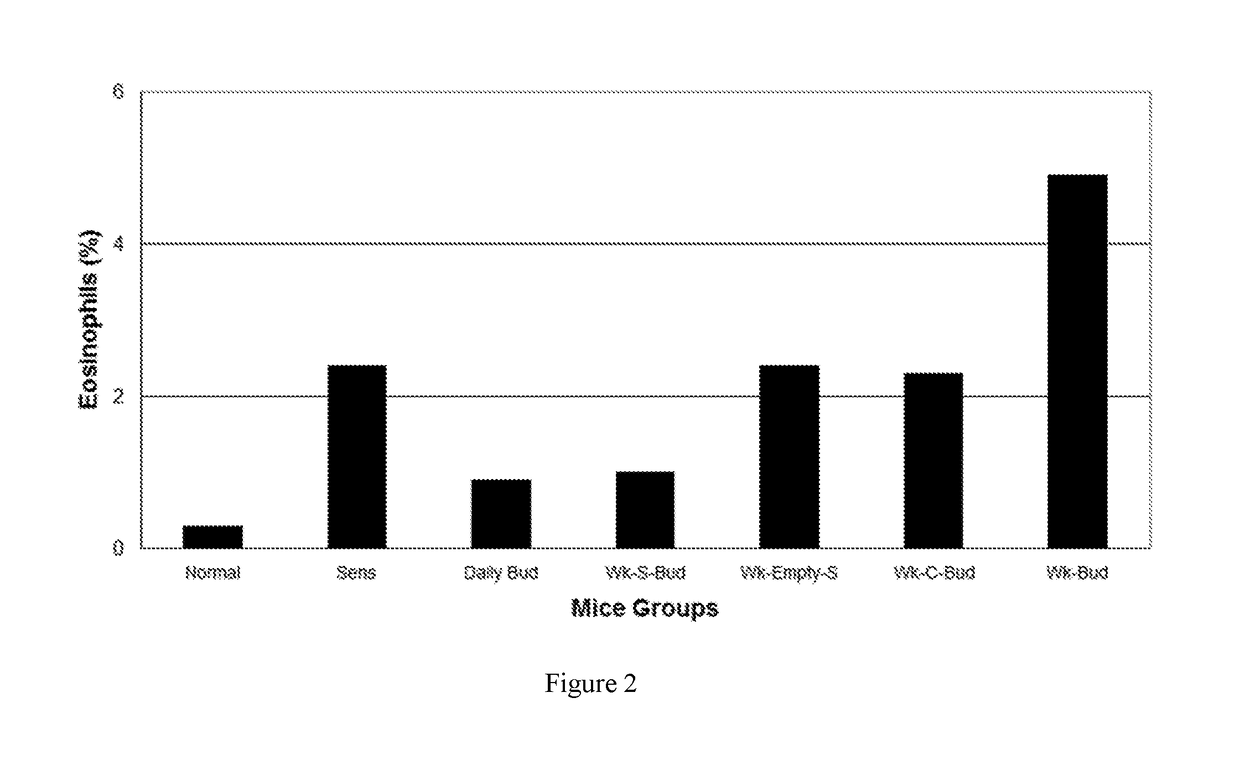
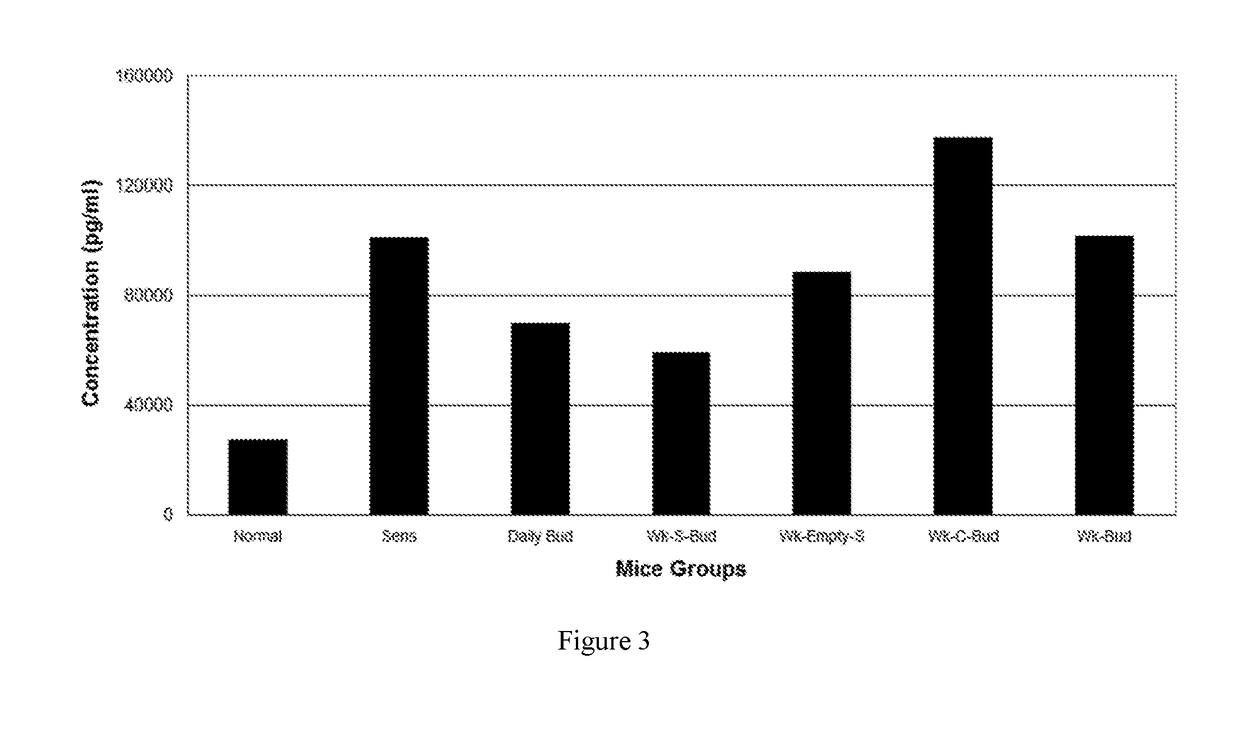
BUD 1: TREATMENT GROUPSNORMALUnsensitized, Untreated Normal miceSENSSensitized, Untreated miceDaily BUD20 μg of budesonide without Carrier given daily-StandardtherapyWK-S-BUD20 μg of budesonide in the Carrier given once a weekWK-C-BUD20 μg of budesonide encapsulated in Conventional Carriergiven once a weekWK-ESBuffer loaded empty Carrier without drug given oncea weekWK-BUD20 μg of budesonide without Carrier given once a weekBUD 1: RESULTSPB EosIgE levels(FIG. 2)(FIG. 3)NORMAL——SENS↑↑Daily BUD↓↓WK-S-BUD↓↓WK-C-BUDØØWK-ESØØWK-BUDØØLegend↑Ø↓—Moderate-SevereNo significantSignificantNoinflammationreduction inreduction ininflammationinflammationinflammation
[0179]In the set of data given for BUD 1, it was demonstrated that one dose of budesonide (BUD) encapsulated in the carrier given once a week (WK-S-BUD), reduced inflammation as effectively as the same dosage of BUD given once a day (Daily BUD) when compared to the Sensitize...
example 3
mparison of BUD in the Carrier with Free Drug / Free Carrier Administered Simultaneously
[0180]
BUD 2: TREATMENT GROUPSNORMALUnsensitized, Untreated Normal miceSENSSensitized, Untreated miceDaily BUD20 μg of budesonide without the Carrier given daily-Standard therapyWK-S-BUD20 μg of budesonide in the Carrier given oncea weekWK-ESBuffer loaded Empty Carrier without drug given oncea weekWK-BUD20 μg of budesonide without the Carrier given oncea weekWK-BUD & ESWK-ES and WK-BUD without encapsulation in theCarrier, given once a weekBUD 2: RESULTSPB EosIgE levels(FIG. 4)(FIG. 5)NORMAL——SENS↑↑Daily BUD↓↓WK-S-BUD↓↓WK-ESØØWK-BUDØØWK-BUD & ESØØ
[0181]In the set of data given for BUD 2, it was demonstrated that one dose of budesonide (BUD) encapsulated in the Carrier given once a week (WK-S-BUD), reduced inflammation as effectively as the same dosage of BUD given once a day (Daily BUD) when compared to the Sensitized Untreated group (SENS) and was comparable to the NORMAL group. Weekly treatments wi...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com