Targeting tumor cells with chemotherapeutic agents conjugated to Anti-matriptase antibodies by in vivo cleavable linking moieties
a technology of in vivo cleavage and binding moieties, which is applied in the field of new immunoconjugates comprising an anticancer agent and a monoclonal antibody, can solve the problems of limiting the clinical value of a stand-alone compound, poor outcome for breast cancer patients, and high cytotoxicity, and achieves less toxicity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
[0186]Expression of Matriptase in Multiple Myeloma and B-Cell Lymphoma Cells
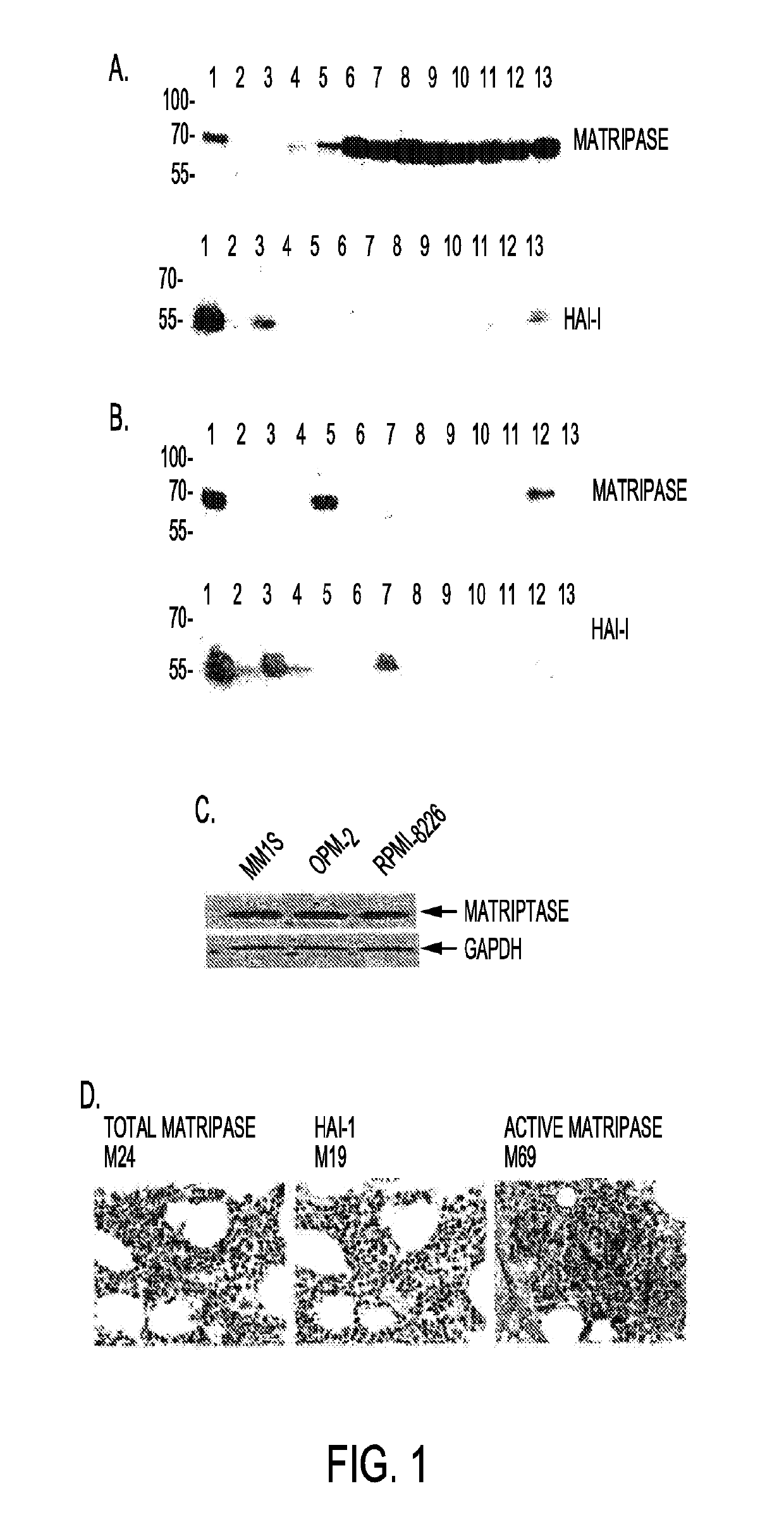
[0187]A panel of 24 cell lines of human hematopoietic malignancies was analyzed by Western blotting for matriptase as well as its endogenous inhibitor HGF activator inhibitor-1 or HAI-1 (FIG. 1). Cell lysates were prepared from 24 hematopoietic cancer cells (lanes 2-14) and T-47D breast cancer cells (lane 1). Equal amounts of proteins were resolved by SDS-PAGE. Levels of matriptase and HAI-1 were assessed by immunoblot analysesusing an anti-matriptase mAb and anti-HAI-1 mAb as indicated (FIGS. 1A and 1B).
[0188]Matriptase was expressed in the majority of the B-cell lymphomas as well as in multiple myeloma cells, indicating that the protease is an important marker for these cancers. In contrast, HAI-1 levels in lymphoma and myeloma appear to be much lower than those in carcinomas, and HAI-1 expression is often lost in highly aggressive lymphomas (Table 1).
[0189]To further verify the expression of matriptase in...
example 2
[0191]Preparation of DOX-Immunoconjugate
[0192]Monoclonal antibodies against matriptase (M24 or M69, obtained from C Y Lin, University of Maryland, Baltimore, Md.; Lin C. Y., et al., J. Biol. Chem., 1999, 274 (26):18237-18242; Chen, Y. W., et al. J. Biol. Chem., 2010, 285 (41):31755-31762) was conjugated to DOX (Sigma) by using Protein-Protein Coupling kit (Invitrogen) according to the manufacture's instruction with some minor modification. Briefly, DOX was reacted with crosslinker SMCC (succinimidyl-4-(N-maleimidomethyl)cyclohexane-1-carboxylate) at a molar ratio (MR) between 1:3 and 1:1.5 (DOX:SMCC) for 1 hour at room temperature with constant stiffing. About 200 mg M24 mAb was reacted with SPDP (Succinimidyl 3-(2-pyridyldithio)-propionate) for 1 hour at room temperature followed by exchange of phosphate buffer using Centrifugal Filter Device (Ultrafree, Millipore). Alternatively, the mAb can be separated from free SPDP by gel filtration with Sephadex G50. DOX maleimide derivative ...
example 3
Cytotoxicity of M24-DOX Toward Multiple Myeloma Cells
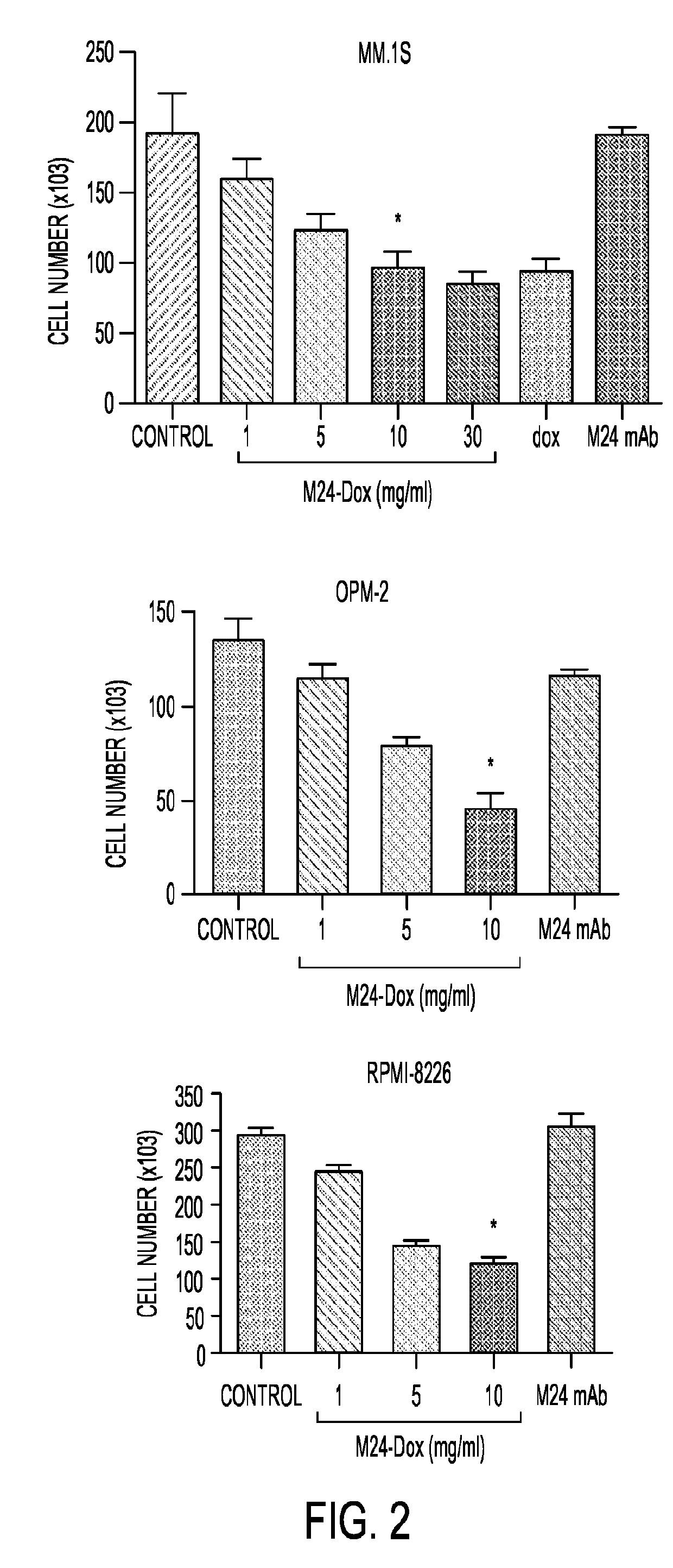
[0195]To assess the cytotoxic effect of the immunoconjugate, MM cells were treated with the conjugated DOX at various concentrations for 48 hours. The immunoconjugate inhibited cell proliferation in a dose-dependent manner. The EC50 of the immunoconjugate was 5 μg / ml (protein). This cytotoxic effect was also observed with treatment of free DOX at 200 nM. As the concentration of M24-DOX at 5 μg / ml is equivalent to 250 nM of free DOX based on the intensity of fluorescence, the potency of the conjugated DOX is therefore similar to the free drug. Exposure of the cells to unmodified antibody had no effect on cell growth, demonstrating that the M24-DOX induced cytotoxic effect is mediated through DOX activity. Two other MM cell lines including OPM-2 and RPMI-8226 were also tested for the cytotoxic activity of M24-DOX as shown in FIG. 2 where MM cells (1×105 / well) in 24-well plates were treated with or without M24-DOX at varying concentr...
PUM
| Property | Measurement | Unit |
|---|---|---|
| weight | aaaaa | aaaaa |
| weight | aaaaa | aaaaa |
| weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com